American scientists have found a way of using bacteria to convert solar energy into liquid fuel. The researchers from Harvard Medical School (MHS), Harvard University’s Faculty of Arts and Sciences, and the Wyss Institute for Biologically Inspired Engineering call the system “a bionic leaf”.
Their findings have been published in the academic journal Proceedings of the National Academy of Sciences (PNAS), February 9 issue.
Being able to harvest sunlight is something humans are just starting to learn about, while plants mastered the art over one billion years ago, using solar energy (sunlight) to feed themselves from the water and air around them in the process we know as photosynthesis.
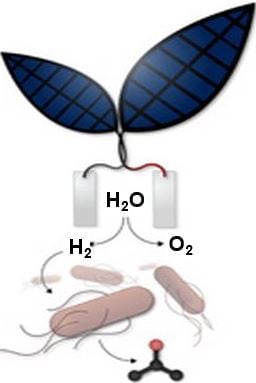
Image credit: Jessica Polka. Harvard Medical School.
Scientists have worked out how to harness solar energy, using electricity from photovoltaic cells to yield hydrogen which can be used in fuel cells later.
However, hydrogen has not caught on as a practical fuel for automobiles or power generation, because our world is designed around liquid fuels.
The “artificial leaf”
In this latest study, the researchers integrated an “artificial leaf” which utilizes a catalyst to make sunlight split water into oxygen and hydrogen, with a bacterium genetically engineered to convert CO2 plus hydrogen into the liquid fuel isopropanol. Isopropanol (C3H8O or C3H7OH or CH3CHOHCH3) is the simplest example of a secondary alcohol.
Professor of Biochemistry and Systems Biology, Pamela Silver, dubbed the system a “bionic leaf”, a nod to the artificial leaf invented by the study’s senior author Professor of Energy Daniel Nocera.
Professor Silver, who is a founding core faculty member of the Wyss, said:
“This is a proof of concept that you can have a way of harvesting solar energy and storing it in the form of a liquid fuel. Dan’s formidable discovery of the catalyst really set this off, and we had a mission of wanting to interface some kinds of organisms with the harvesting of solar energy. It was a perfect match.”
Sparked by an interest in “personalized energy”
Professors Nocera and Silver started collaborating two years ago, soon after Nocera moved from MIT to Harvard. They were both interested in “personalized energy,” i.e. making energy locally, in contrast to the current system, for example oil, which relies on central production, and is delivered as gas (UK English: petrol) in filling stations.
Local energy, they believed, would be an attractive option in the emerging economies.
Prof. Silver said:
“It’s not like we’re trying to make some super-convoluted system. Instead, we are looking for simplicity and ease of use.”
Prof. Nocera’s artificial leaf relies on catalysts made from cheap and readily accessible materials.
Prof. Nocera said:
“The catalysts I made are extremely well adapted and compatible with the growth conditions you need for living organisms like a bacterium.”
In this new system, as soon as the artificial leaf has produced hydrogen and oxygen, the hydrogen is fed to a bacterium called Ralstonia eutropha. An enzyme takes the hydrogen back to electrons and protons, then combines them with CO2 to replicate – making more cells.
Then, based on previous discoveries made by MIT professor Anthony Sinskey, new pathways in the bacterium are metabolically engineered to produce isopropanol.
Co-author Brendan Colón, a graduate student in systems biology in the Silver lab, said:
“The advantage of interfacing the inorganic catalyst with biology is you have an unprecedented platform for chemical synthesis that you don’t have with inorganic catalysts alone. Solar-to-chemical production is the heart of this paper, and so far we’ve been using plants for that, but we are using the unprecedented ability of biology to make lots of compounds.”
Prof. Silver said the same principles could be used to produce vitamins in small quantities.
Aiming to improve efficiency
The scientists’ next challenge is to increase the bionic leaf’s ability to translate sunlight into biomass by optimizing the catalyst and the bacteria. They are aiming for 5% efficiency, versus nature’s 1% efficiency for photosynthesis to turn solar energy into biomass.
Prof. Nocera said:
“We’re almost at a 1 percent efficiency rate of converting sunlight into isopropanol. There have been 2.6 billion years of evolution, and Pam and I working together a year and a half have already achieved the efficiency of photosynthesis.”
The study was funded jointly by the Air Force Office of Scientific Research, the Office of Naval Research, and the National Science Foundation.
Citation: “Efficient solar-to-fuels production from a hybrid microbial–water-splitting catalyst system,” Joseph P. Torella, Christopher J. Gagliardi, Janice S. Chen, D. Kwabena Bediako, Brendan Colón, Jeffery C. Way, Pamela A. Silver, and Daniel G. Nocera. PNAS. Feb 9, 2015. DOI: 10.1073/pnas.1424872112.

