The Martian surface may be much more toxic for human life than previously thought, a team of scientists from the University of Edinburgh warns after carrying out a series of experiments. They have investigated the behaviour of ‘perchlorates’, chemical compounds that are found on Martian surface.
When exposed to ultraviolet (UV) light while in Mars-like conditions, the perchlorates can kill bacteria that are commonly found in spacecraft cabins.
The researchers believe that their findings may have implications for potential contamination from human and robotic Mars explorations.
The scientists wrote about their study and findings in the prestigious Nature journal Scientific Reports 7 (citation at bottom of page).
What are perchlorates?
Perchlorates are reactive chemicals. They were first detected in arctic Martian soil by NASA’s Phoenix lander, which landed on the Red Planet nine years ago.
Perchlorate salts are principally used by us as propellants, because they are extremely powerful oxidizing agents.
Perchlorate contamination in Earth’s environment has been studied extensively. Studies have shown that they are harmful to humans, including damage to the thyroid gland.
 According to NASA, the presence of perchlorates on the Martian surface may pose a significant hazard but also a useful resource. (Image: nasa.gov)
According to NASA, the presence of perchlorates on the Martian surface may pose a significant hazard but also a useful resource. (Image: nasa.gov)
The following quote comes from an article published by Scientific American:
“Perchlorate in the environment is a health concern because it can disrupt the thyroid’s ability to produce hormones needed for normal growth and development.”
“Besides its potential to cause endocrine system and reproductive problems, perchlorate is considered a ‘likely human carcinogen’ by the U.S. Environmental Protection Agency (EPA). Some 11 million Americans live in areas where concentrations of perchlorate in public drinking water supplies are significantly higher than what is considered safe.”
Perchlorates on Martian surface
The Scottish scientists say that their study also suggests that the effect of perchlorates can be compounded by iron oxides and hydrogen peroxide – two types of chemicals found on the surface of the Red Planet.
In experiments where perchlorates, iron oxides, and hydrogen peroxide were present, bacterial cells were more likely to die by a factor of ten compared with perchlorates alone.
Researchers have speculated what the effects of perchlorates might be on the habitability of Martian surface since they were discovered there.
Scientists at the UK Centre for Astrobiology and School of Physics and Astronomy have carried out extensive research on the potential reactivity of perchlorates and their effect on one bacterium – Bacillus subtilis – which is common in soils and rocks on Earth, and is also found on spacecraft.
Their studies showed that when magnesium perchlorate was exposed to ultraviolet radiation similar to what is found on the Martian surface, it became a super-effective killer of bacteria – much more deadly than UV light alone.
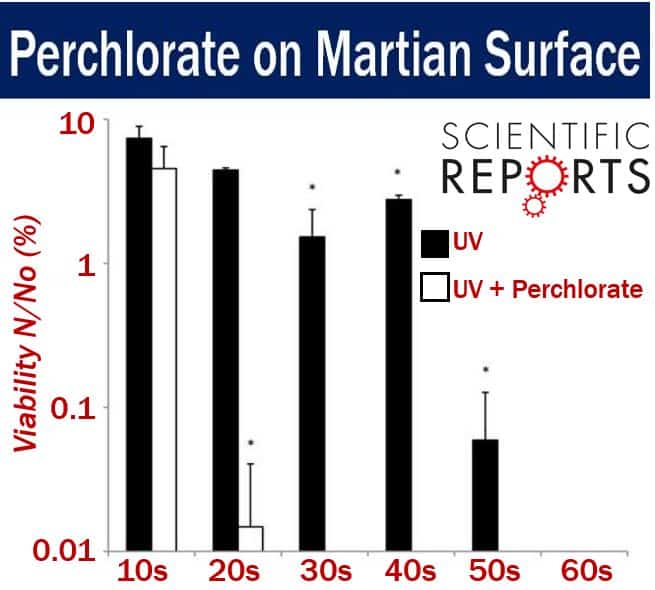 Effects of UCV-irradiated Mg(ClO4)2 on Bacillus subtilis viability. (Image: adapted from nature.com)
Effects of UCV-irradiated Mg(ClO4)2 on Bacillus subtilis viability. (Image: adapted from nature.com)
Scientists have suspected for some time that the Martian surface may be toxic for humans. What this latest study demonstrates is that it may be extremely damaging to living cells.
This is due to a lethal mix of perchlorates, iron oxides, oxidants, and ultraviolet radiation.
In an Abstract preceding the main article in the journal, the authors wrote:
“These data show that the combined effects of at least three components of the Martian surface, activated by surface photochemistry, render the present-day surface more uninhabitable than previously thought, and demonstrate the low probability of survival of biological contaminants released from robotic and human exploration missions.”
Citation:
‘Perchlorates on Mars enhance the bacteriocidal effects of UV light‘, Jennifer Wadsworth and Charles S. Cockell, Scientific Reports 7, Article Number: 4662 (2017). DOI: 10.1038/s41598-017-04910-3. Published online 06 July, 2017.

