They start out tiny and strange, grown from stem cells floating in special liquids. These organoids look like little blobs, but to many researchers, they represent hope. Hope for treating diseases that once felt impossible to tackle. Hope for predicting how certain tissues will behave before they form fully. Now, scientists in Japan say they’ve built a tool that can spot which organoids will thrive — long before the final shape emerges.
A team from Kyushu University and Nagoya University has used artificial intelligence to judge organoid development in the lab. Their study, published this month, focused on hypothalamic-pituitary organoids. These mini-tissues produce crucial hormones like ACTH, a key player in managing stress, metabolism, and more. Problems with ACTH can cause serious illness. “In our lab, our studies on mice show that transplanting hypothalamic-pituitary organoids has the potential to treat ACTH deficiency in humans,” says Associate Professor Hidetaka Suga, from Nagoya University’s Graduate School of Medicine.
The researchers found that a protein called RAX can hint at how well the organoid will do. Early, wide expression of RAX usually means better hormone production later. But there’s a catch: organoids intended for human use can’t be genetically modified to glow with fluorescent proteins for easy tracking. That left experts staring at plain images, trying to decide which lumps would become quality tissue. This human judgment took time and wasn’t always right.
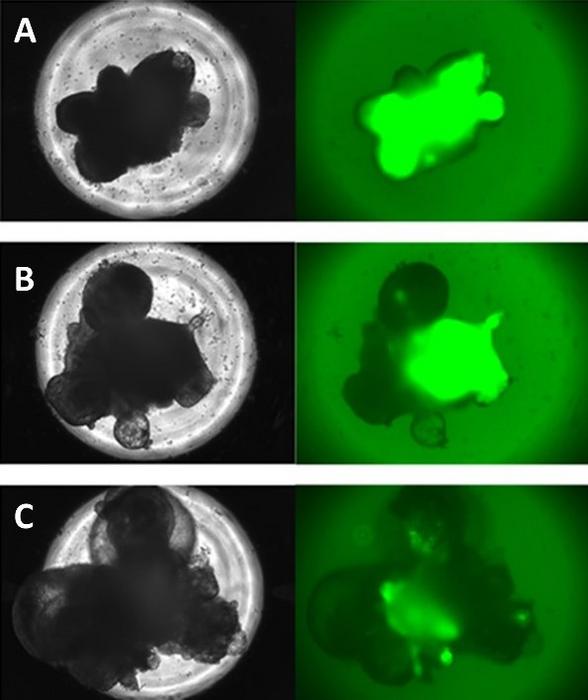
Enter the AI. “Deep-learning models are a type of AI that mimics the way the human brain processes information, allowing them to analyze and categorize large amounts of data by recognizing patterns,” explains Professor Hirohiko Niioka of Kyushu University’s Data-Driven Innovation Initiative. The team trained their models on a set of images. Some of the organoids had been made to glow, revealing RAX expression, while others were left normal. The model learned to predict how these tiny clumps would turn out. After comparing the AI’s calls to expert guesses, the results were clear. “The deep-learning models outperformed the experts in all respects: in their accuracy, their sensitivity, and in their speed,” says Niioka.
Then they tested the AI on organoids with no special glowing tags. The model’s predictions matched real-world outcomes. Those it called “high quality” ended up rich in RAX and later showed strong ACTH production. Those it labeled as lower quality did poorly. “As far as we know, this is the first time in the world that deep-learning has been used to predict the future of organoid development,” says Niioka.
For researchers, this breakthrough means better odds of selecting the right organoids early on. It can help save time and money. It can guide which organoids might be best for future treatments. “We can quickly and easily select high-quality organoids for transplantation and disease modeling, and reduce time and costs by identifying and removing organoids that are developing less well,” concludes Suga.
This step forward may not fix every problem overnight. But it marks a real shift in how scientists grow and judge these promising mini-tissues. Organoids have always felt a bit mysterious. Now, with a smarter pair of eyes, that mystery feels a little easier to manage.
