A lithium-ion battery that never burns or explodes – it shuts down when the temperature is too high and restarts when it cools down – has been created by a team of scientists from Stanford University in California, USA.
Their new technology could prevent devices from catching fire. Over the past year, several battery-operated devices have been recalled because of a battery fire risk, including hoverboards, navigation systems, computers and recliners.
Electronic cigarettes, which are known to blow up and cause fires if recharged incorrectly, could also become hazard-free.
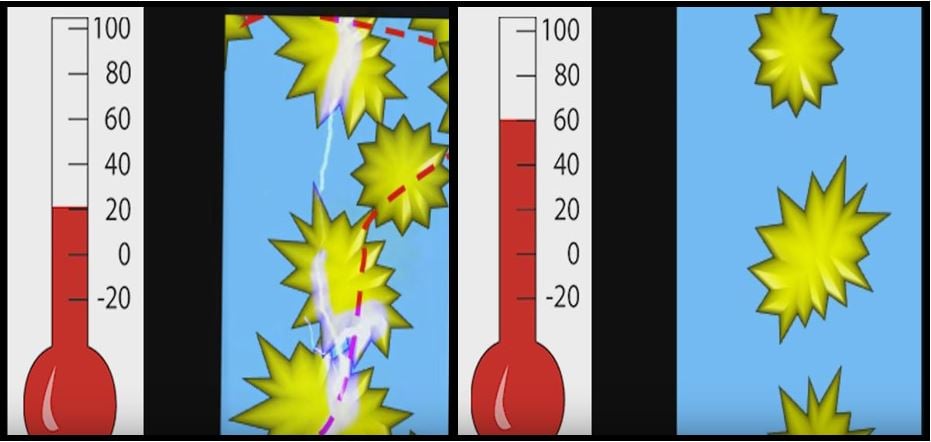 When the spikes are close to each other (touching) there will be electricity going through the polymer. When the battery heats up the polymer expands, pulling the spiky particles apart, and electricity can no longer flow. (Image: Stanford Precourt Institute for Energy)
When the spikes are close to each other (touching) there will be electricity going through the polymer. When the battery heats up the polymer expands, pulling the spiky particles apart, and electricity can no longer flow. (Image: Stanford Precourt Institute for Energy)
The first battery that shuts down when it gets hot
Zhenan Bao, Professor of Chemical Engineering and by courtesy, Professor of Chemistry & Professor of Materials Science and Engineering at Stanford University, said:
“People have tried different strategies to solve the problem of accidental fires in lithium-ion batteries. We’ve designed the first battery that can be shut down and revived over repeated heating and cooling cycles without compromising performance.”
Prof. Bao and colleagues wrote about their new battery and how they created it in an article published in the academic journal Nature Energy.
Typical lithium-ion batteries consist of two electrodes and a liquid (or gel) electrolyte that carries charged particles between them.
When the battery is overcharged, shorted or punctured it gets hot. If the battery’s temperature exceeds 300 °F (150 °C), the electrolyte may catch fire and cause an explosion.
Scientists have tried out a number of techniques to prevent batteries from catching fire or exploding, including adding flame retardants to the electrolyte. Two years ago, Yi Cui, Associate Professor of Material Science Engineering at Stanford, a David Filo and Jerry Young Faculty Scholar, created a ‘smart’ battery that gives plenty of warning before it overheats.
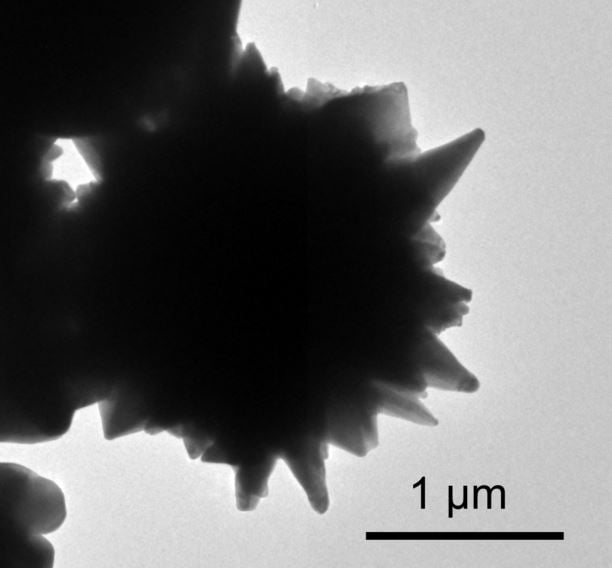 One of the spiky nanoparticles of graphene-coated nickel. They control the flow of electricity as the battery heats up and cools (1µ =1 micrometer or 1×10-6 of one metre). (Image: energy.stanford.edu. Credit: Zheng Chen)
One of the spiky nanoparticles of graphene-coated nickel. They control the flow of electricity as the battery heats up and cools (1µ =1 micrometer or 1×10-6 of one metre). (Image: energy.stanford.edu. Credit: Zheng Chen)
Prof. Cui, a co-author, said:
“Unfortunately, these techniques are irreversible, so the battery is no longer functional after it overheats. Clearly, in spite of the many efforts made thus far, battery safety remains an important concern and requires a new approach.”
Nanospikes that separate when it’s too hot
Prof. Bao and postdoctoral scholar Zheng Chen turned to nanotechnology to address the problem. Prof. Bao recently invented a wearable sensor that monitors the temperature of the human body. It is made of a plastic material embedded with miniature particles of nickel with nanoscale spikes sticking out from their surface.
In the experiment with a battery, the scientists coated the spiky nickel particles with graphene, a super-thin (atom thick) layer of carbon, and embedded the particles in a thin film of elastic polyethylene.
 With this new technology, batteries would not longer be a fire/explosion risk.
With this new technology, batteries would not longer be a fire/explosion risk.
Lead author, Dr. Chen, said:
“We attached the polyethylene film to one of the battery electrodes so that an electric current could flow through it. To conduct electricity, the spiky particles have to physically touch one another. But during thermal expansion, polyethylene stretches.”
“That causes the particles to spread apart, making the film nonconductive so that electricity can no longer flow through the battery.”
When the battery’s temperature exceeded 160 °F (70°C), the polyethylene film rapidly expanded like a balloon, which made the spiky particles separate, and the battery shut down.
However, when the temperature was below 160 °F again, the polyethylene shrank, the spikes came back into contact, and the battery started working (generating electricity) again.
Prof. Bao said:
“We can even tune the temperature higher or lower depending on how many particles we put in or what type of polymer materials we choose. For example, we might want the battery to shut down at 50 C or 100 C.”
Test repeated several times
To determine the stability of the new material, Prof. Bao and colleagues applied heat to the battery with a hot-air gun several times. Every time, the battery shut down when it got too hot and rapidly started operating again when the temperature cooled.
Prof. Cui said:
“Compared with previous approaches, our design provides a reliable, fast, reversible strategy that can achieve both high battery performance and improved safety,” Cui said. “This strategy holds great promise for practical battery applications.”
In an Abstract in the journal the authors wrote:
“Batteries with this self-regulating material built in the electrode can rapidly shut down under abnormal conditions such as overheating and shorting, and are able to resume their normal function without performance compromise or detrimental thermal runaway. Our approach offers 103–104 times higher sensitivity to temperature changes than previous switching devices.”
Citation: “Fast and reversible thermoresponsive polymer switching materials for safer batteries,” Zheng Chen, Po-Chun Hsu, Jeffrey Lopez, Yuzhang Li, John W. F. To, Nan Liu, Chao Wang, Sean C. Andrews, Jia Liu, Yi Cui & Zhenan Bao. Nature Energy. 11 January 2015. DOI: 10.1038/nenergy.2015.9.
Video – A lithium-ion battery that does not burn
This Stanford video explains how the lithium-battery shuts down before overheating, and restarts as soon as the temperature has cooled.
