Nuclear fission and nuclear fusion are two different reactions that release vast amounts of energy locked in the nuclei of atoms.
In both reactions some mass is lost and converted to energy according to Einstein’s famous e=mc² equation.
However apart from this similarity, these reactions are very different.
Simply put, nuclear fission is where the nucleus of a heavy atom splits into two lighter ones, and fusion is where two light nuclei combine to form a bigger one.
Fission is the process currently used in nuclear reactors to make electricity, while fusion is still at the experimental stage – with international teams working hard to find ways to control the reaction in a contained space. Our sun is a giant fusion reactor: every second it transforms 600 million tons of hydrogen into helium.
However, many scientists believe it is only a matter of time before we overcome the barriers to creating viable fusion technology for energy production, and when that time comes, fusion will offer many advantages over fission, for instance it creates less radioactive products, and uses a virtually unlimited fuel supply – heavy hydrogen, which is abundant in seawater.
Differences between nuclear fission and nuclear fusion
- Fission starts with a large nucleus and finishes with two smaller ones, whereas fusion starts with two small nuclei and ends with a larger one.
- To produce a fission reaction you need a critical mass of the starting substance plus high-speed neutrons, whereas to produce a fusion reaction you need very high temperatures and high density of the starting substance.
- Fission does not usually occur in nature, whereas fusion occurs in stars like our sun.
- Fission is used in nuclear reactors to produce electricity, whereas fusion is still at the experimental stage.
- Fission produces many highly radioactive products with a long half life, whereas fusion does not. (However, one way to produce a fusion reaction uses a fission ‘trigger’, and that does produce radioactive substances.)
- Fission as a means of producing power uses uranium as the primary fuel, of which there is a large but limited supply, whereas fusion, when the technology becomes viable, is likely to use heavy hydrogen, which is abundant.
- While the energy released by splitting one uranium atom is millions of times more than the amount of energy released in burning an atom of carbon, the energy released in a fusion reaction is even greater.
How can both splitting and combining nuclei release energy?
One puzzling question that often arises when trying to understand the difference between fission and fusion is, if splitting a nucleus releases energy, then how can combining two nuclei also release energy?
This begins to make sense when we look into how binding energy works in nuclei.
Nuclear binding energy is the energy you would have to put in to completely split a nucleus up into its component protons and neutrons. In a sense it is a measure of how tightly bound a nucleus is – the less binding energy per proton or neutron in the nucleus, the more tightly bound the nucleus. And this is different in differently-sized nuclei.
And there is a very simple rule in nuclear physics:
If a nuclear reaction produces nuclei that are more tightly bound than the originals – i.e. with lower binding energy per neutron or proton – then it releases energy.
Take a typical fission reaction used in nuclear power generation, where a uranium nucleus splits into barium and krypton nuclei. The barium and krypton nuclei are more tightly bound than the original uranium nucleus.
Equally, in the fusion reaction where two heavy hydrogen nuclei combine to form a helium nucleus, the helium nucleus is more tightly bound than the heavy hydrogen nuclei. (Heavy hydrogen is a term given to ‘isotopes’ of hydrogen that have one proton, like normal hydrogen, but unlike normal hydrogen they also have one or two neutrons.)
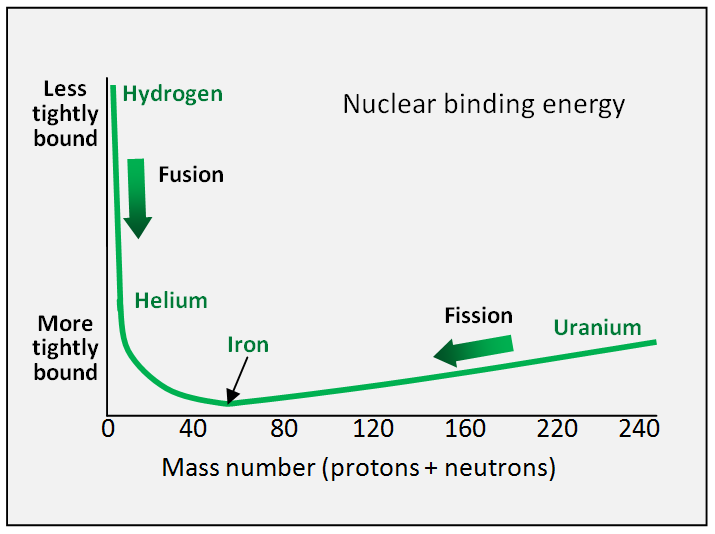
Of all the elements, iron has the most tightly bound nuclei, with 26 protons. If you plot a graph of how tightly bound the nucleus of each element is against its atomic mass (number of protons plus neutrons), it looks like this:
A nuclear binding energy curve showing iron, with the lowest average binding energy per nucleon (proton or neutron), has the most tightly bound nucleus.
Iron sits in the bottom of a ‘valley’, with a steep slope on one side and a shallow slope on the other. The steep slope goes up to hydrogen, and the shallow slope goes up to uranium and beyond. The further into the bottom of the valley you go, closer to iron, the tighter the bonds in the nucleus, and the less binding energy per neutron or proton.
So, as long as the products of your nuclear reaction shift towards iron in the bottom of the valley, you are going to release energy. Thus hydrogen fusing to make helium (moves toward iron down the steep slope) and uranium splitting into krypton and barium (moves towards iron down the shallow slope) both release energy.
Further reading: