Engineers from Columbia University in New York City have developed a solar rig that uses energy from the sun to extract hydrogen from seawater and could one day operate as a floating platform out on the ocean.
The solar rig uses electrolysis – a technique that breaks compounds down into simpler substances by passing an electric current through them – to separate water (H2O) into hydrogen (H2) and oxygen (O2) gases.
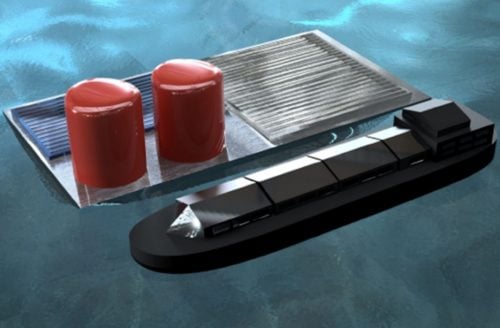 This computer-generated image shows how the floating solar rig might look out in the ocean.
This computer-generated image shows how the floating solar rig might look out in the ocean.
Image: Justin Bui/Columbia Engineering
The electricity for the electrolyzer comes from the rig’s on-board photovoltaic (PV) cells that convert solar energy into electricity by using photons to knock electrons out of atoms so that they are free to move and create an electric current.
No membrane required to ensure hydrogen purity
The Columbia University engineers say that the present “state-of-the-art electrolyzers” that are used to produce hydrogen fuel from water need an expensive and delicate membrane to ensure a high level of purity of the extracted H2.
But their solar rig can ensure a high level of H2 purity without the need for a membrane.
Once scaled up to industrial size, such a solar rig should not only be less expensive but also more robust and therefore able to operate in open seawater, they argue.
Daniel Esposito, assistant professor of chemical engineering, who has been studying how to produce storable hydrogen fuel from water using electrolysis, heads the group that is working on the new solar rig.
He says that they are “very excited” about the potential of solar fuel technologies “because of the tremendous amount of solar energy that is available.”
“Being able to safely demonstrate a device that can perform electrolysis without a membrane brings us another step closer to making seawater electrolysis possible,” adds Jack Davis, a PhD student on Prof. Esposito’s team and lead author of a scientific report about their work that was published recently in the International Journal of Hydrogen Energy.
In that study paper, the team describes how it developed and tested the “floating membraneless PV-electrolyzer” solar rig as a means to produce clean hydrogen fuel at an acceptable level of purity.
Emissions-free clean hydrogen fuel
There are great hopes that hydrogen – which NASA has been using as a rocket fuel since 1958 – will be an important sustainable and clean fuel in the future, if a viable way to extract it free of carbon dioxide (CO2) emissions can be found.
There is a growing demand for hydrogen as a road transport fuel as the market for fuel cell electric vehicles expands.
Most of the hydrogen fuel that is in use today comes from “steam methane reforming,” a process that extracts H2 from natural gas by passing steam through it.
However, this method is not emissions-free because it also releases CO2; the methane (CH4) plus steam (H2O) becomes hydrogen (H2) and carbon dioxide (CO2).
Electrolysis of water using electricity from solar PV cells is seen as a greener alternative way of making hydrogen fuel because it does not produce CO2.
‘Buoyancy-induced separation’ of hydrogen
Prof. Esposito and his team used a method called “buoyancy-induced separation” to produce hydrogen with a high degree of purity without the need for a membrane.
They developed a unique type of “flow-through mesh electrode” with a special platinum coating that acts as the catalyst for the electrolysis reaction that breaks down the H2O.
For the electrolysis to work, there have to be two electrodes (a positive for the O2 and a negative for the H2) quite close to each other (2mm distance).
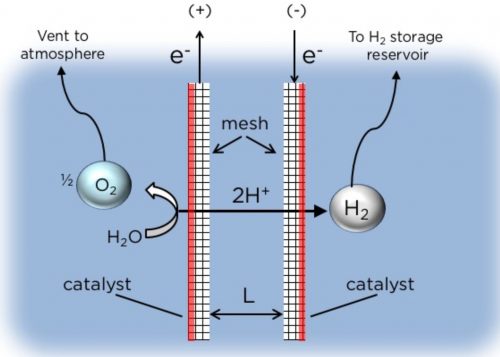 In the membraneless design, the two mesh electrodes, separated by a narrow gap, each generates O2 and H2 gases as the electric current passes through the water.
In the membraneless design, the two mesh electrodes, separated by a narrow gap, each generates O2 and H2 gases as the electric current passes through the water.
Image: Daniel Esposito/Columbia Engineering
The H2 and O2 gases form on the “platinum electrocatalyst” coating as bubbles. When the bubbles are full enough – that is, they reach their buoyancy point – they float upwards and the H2 is collected in a chamber while the O2 can be either collected in a chamber or “vented into the atmosphere.”
At first, the team experimented with coating both the outsides and the insides of the electrode pair, but this resulted in too many O2 bubbles crossing over into the path of the H2 bubbles to their collection chamber; the collected H2 was only 93 percent pure.
But, when they experimented with coating just the outsides of the electrode pair, the engineers found that they could collect the H2 at 99 percent purity – there was so little crossing over of O2 bubbles into the path of the H2 bubbles.
‘Artificial photosynthesis’
The engineers are now improving the design of the solar rig to optimize its efficiency for use with real seawater, which is chemically different to the more “ideal” water-based electrolytes they have been experimenting with in the laboratory.
They are also hoping to develop modular versions of the solar rig that can be more easily scaled up for deployment at sea.
“These solar fuels generators are essentially artificial photosynthesis systems, doing the same thing that plants do with photosynthesis, so our device may open up all kinds of opportunities to generate clean, renewable energy,” says Davis.
“Our challenge,” Prof. Esposito adds, “is to find scalable and economical technologies that convert sunlight into a useful form of energy that can also be stored for times when the sun is not shining.”
Video showing the H2 and O2 bubbles coming off the electrodes
The following video from Columbia University shows the upward ascent of the H2 and O2 bubbles as they leave the surfaces of the electrodes in the solar rig.
Note how the design in which the catalyst coating is only on the outsides of the electrode pair (“asymmetric electrodes,” on the left) results in fewer O2 bubbles drifting into the upward path of the H2 bubbles.
