What are clinical trials? Definition and examples
We call medical experiments involving human beings clinical trials. Clinical trials contrast with animal testing or in vivo testing, which uses non-human animals.
Researchers carry out clinical trials to determine whether a drug, device, or therapy effective for humans. They also use them to determine whether it is safe.
According to the National Heart, Lung, and Blood Institute, part of the National Institutes for Health (NIH):
“Clinical trials are research studies that explore whether a medical strategy, treatment, or device is safe and effective for humans.
“These studies also may show which medical approaches work best for certain illnesses or groups of people. Clinical trials produce the best data available for health care decision making.”
Processes before clinical trials
The aim of clinical trials is research. Therefore, the studies must follow strict scientific guidelines or procedures.
Animal testing
Typically, the process starts in the lab, where researchers first develop an active ingredient, device, or idea. The active ingredient is the part of a medication that is biologically active.
If, for example, a substance shows promise, the scientists move onto the next step. The next step is animal testing.
Animal testing determines whether the substance has an effect on a living body. It can also tell us whether it is harmful.
Even if it works well on animals, that is no guarantee that it will do the same with humans. Although humans are animals, not all animals respond to substances in the same way.
From animal testing to clinical trials
To determine whether something is effective and safe for humans, we need to carry out clinical trials. In other words, we need to test it on human beings.
Trials on humans typically begin with very small groups for safety purposes. At this stage, researchers need to determine whether whatever they are testing harms humans.
During the later phases of clinical trials, scientists learn more about its risks and benefits.
Clinical trials – three possibilities
Clinical trials may determine that, for example, a new substance:
– Helps patients by reducing the severity of their symptoms, curing them, or improving their outcomes.
– Makes no difference. In other words, neither benefits nor harms patients.
– Has unexpected and undesirable side-effects. We also use the term ‘adverse events.’ In other words, the substance causes unexpected harm.
Clinical trials – cancer therapies
According to Cancer Research UK, clinical trials may look at:
– Causes and risks – how lifestyles, genetics, the environment, and other factors may raise a human’s risk of developing cancer.
– Prevention – using medications to reduce the risk of developing cancer. Trials may also look at lifestyle changes.
– Screening – for high-risk individuals. In other words, people with a higher-than-average risk of developing cancer. If a woman has a mother who had breast cancer, her risk of developing the disease is higher.
– Diagnosis – new scans and tests.
– Treatments – new medications or combinations of them. Also, new ways of administering treatment as well as new types of treatment.
– Reducing symptoms’ severity – new medications or complementary therapies. There may also be ways of reducing the incidence or severity of side effects.
Types of clinical trials – phases
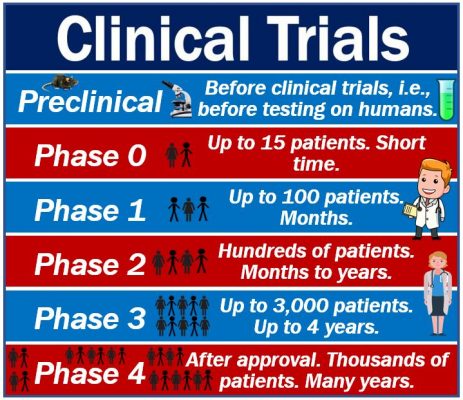
There are typically three main clinical trial phases. There is Phase 1, Phase 2, Phase 3, and Phase 4. Not all trials reach the Phase 4 stage. In some cases, there is also a Phase 0.
We can also write them using Roman numerals. We can write either Phase 4 or Phase IV.
Phase 4 occurs after regulatory authorities, such as the FDA in the USA, have approved the drug. In other words, after the medication has been licensed. Phase 4 gathers information about safety, long-term risks and benefits, and the side effects of the drug.
In the explanations of the Phases below, the words ‘drugs’ or ‘medications’ could be replaced by ‘devices’ or ‘therapies.’
Phase 0
Some clinical trials have a Phase 0. This phase involves a very small number of participants. In most cases, fewer than fifteen patients.
Healthline says the following regarding Phase 0:
“Investigators use a very small dose of medication to make sure it isn’t harmful to humans before they start using it in higher doses for later phases.”
“If the medication acts differently than expected, the investigators will likely to do some additional preclinical research before deciding whether to continue the trial.”
Phase 1
During Phase 1 clinical trials, researchers look at the effect of a drug on between 20 and 100 participants. These are ‘healthy’ volunteers. In other words, they have no underlying health conditions.
The researchers spend several months observing the effects of the drug.
Phase 1 also tries to determine what the highest dose is. In other words, how much of a substance can a human tolerate without serious side effects?
During this phase, researchers are also looking at the administration options, i.e., injection, oral, eye-drops, etc.
The FDA says that most drugs, i.e., 70%, move from Phase 1 to Phase 2. FDA stands for Food and Drug Administration. It is the United State’s regulatory agency in charge of drugs, medical devices, foods, cosmetics, and tobacco products.
Phase 2
Phase 2 clinical trials involve many more patients than Phase 1; usually several hundred. The participants are people who live with the illness or condition that the new drug targets.
This phase can last from several months to two years.
According to the FDA, Phase 2 focuses on the substance’s efficacy and side effects. It also says that about one-third of drugs in Phase 2 move onto Phase 3.
Phase 3
Phase 3 involves between 300 and 3,000 participants. We also call them volunteers. All the volunteers live with the illness or condition that the new medication targets.
Phase 3 trials can last from one to four years. During this phase, researchers focus on the drug’s efficacy as well as adverse reactions.
The FDA informs that between 25% and 30% of new drugs move onto Phase 4.
Phase 4
Phase 4 clinical trials take place after the regulatory authority, such as the FDA, has approved the new drug. This phase typically includes several thousand patients. It tries to identify and evaluate the long-term effects of the new medication. The monitoring may continue for several years.
Accord Clinical Research says the following regarding Phase 4 clinical trials:
“Phase IV research takes place after the FDA approves the marketing of a new drug.”
“Through Phase IV clinical studies, new drugs can be tested continuously to uncover more information about efficacy, safety and side effects after being approved for marketing.”
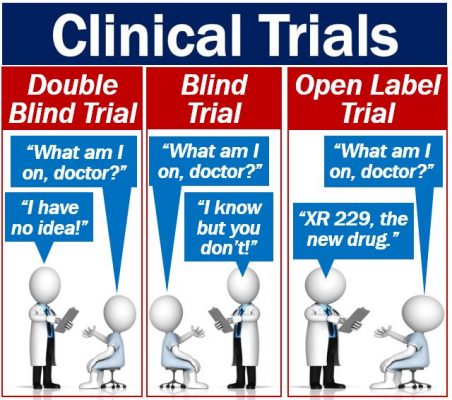
Blind, double-blind, and open-label
There are blind, double-blind, and open-label clinical trials. What do they mean?
Blind trial
In a blind trial, the participants or volunteers do not know whether they are taking the target drug or a placebo. In other words, they do not know which group they are in. The researchers or doctors, on the other hand, do know.
However, clever participants may pick up on subtle clues that the researchers give out unintentionally. We call this ‘observer bias.’ Observer bias can distort the results, i.e., make them unreliable.
The BBC GCSE Bitesize website says the following about doctors and placebos:
“Many doctors do not like giving a placebo to patients with a disease because they feel the patient will not benefit from taking a fake drug and will not get better. They do not think this is fair to the patient.”
Double-blind trial
In double-blind clinical trials, neither the researchers/doctors not the participants know what group people are in. Nobody knows until the end of the trial.
In this type of trial, there is no risk of bias, which makes the results much more reliable. However, setting up a double-blind trial is much more complicated than a blind one.
Open-label trial
In open-label trials, both patient and doctor know what the patient is taking. People often use this type of trial as a last resort. In other words, there is no other treatment, and the doctor believes that the patient won’t recover.
Video – Clinical Trials
This Cancer Research UK video talks about clinical trials. The video begins by introducing John, a cancer patient, who is participating in a cancer research trial.

