Imagine a microscopic wind farm that generates electricity by harnessing the movement of bacteria in a fluid. This is exactly what a team of scientists at Oxford University believe could soon be achieved in real life after carrying out a study.
The researchers explained in the journal Science Advances how they used computer simulations to demonstrate that the random swarming effect of dense active matter such as bacteria could be organised to rotate cylindrical rotors and provide a steady and reliable source of electrical power.
Microscopic wind farm potential for minuscule devices
Co-author Dr Tyler Shendruk, an EMBO long-term fellow in the Rudolf Peierls Centre for Theoretical Physics at the University of Oxford, and colleagues say that these biologically-driven microscopic power plants could eventually become the ultra-tiny engines for minuscule, man-made devices that are self-assembled and self-powered.
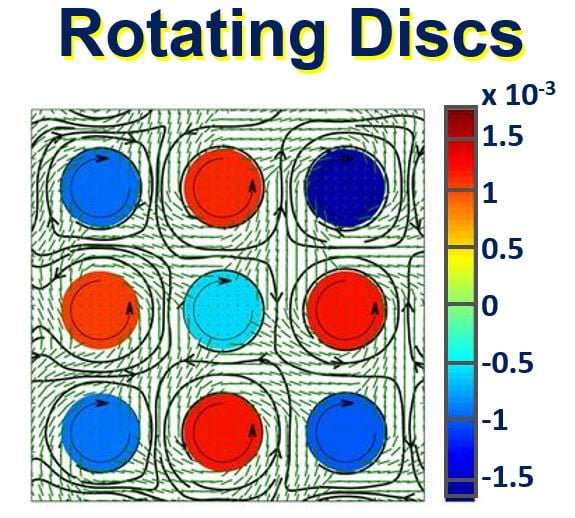 An array of counter-rotating discs in mesoscale turbulence generated by bacteria moving in a fluid. (Image: Science Advances)
An array of counter-rotating discs in mesoscale turbulence generated by bacteria moving in a fluid. (Image: Science Advances)
Dr. Shendruk said:
“Many of society’s energy challenges are on the gigawatt scale, but some are downright microscopic. One potential way to generate tiny amounts of power for micro-machines might be to harvest it directly from biological systems such as bacteria suspensions.”
Dense bacterial suspensions are the perfect example of active fluids that flow chaotically – spontaneously. While swimming bacteria have the capacity to swarm and drive disorganised living flows, they are generally too disordered to extract any useful power from.
However, when the Oxford researchers dipped a lattice of sixty-four symmetric microrotors into this active fluid, they found that the bacteria spontaneously organised themselves in such a way that neighbouring rotors started to spin in opposite directions – much like the blades spread out over a windfarm.
 Wind farms on land harness the energy from the movement of air to generate electricity. Using the same principle with ultra-tiny rotors, we could harness the energy of bacteria moving. (Image: University of Oxford)
Wind farms on land harness the energy from the movement of air to generate electricity. Using the same principle with ultra-tiny rotors, we could harness the energy of bacteria moving. (Image: University of Oxford)
The rotors self-assembled
Regarding the rotors, Dr. Shendruk said:
“The amazing thing is that we didn’t have to pre-design microscopic gear-shaped turbines. The rotors just self-assembled into a sort of bacterial windfarm.”
“When we did the simulation with a single rotor in the bacterial turbulence, it just got kicked around randomly. But when we put an array of rotors in the living fluid, they suddenly formed a regular pattern, with neighbouring rotors spinning in opposite directions.”
Co-author Dr. Amin Doostmohammadi, a Postdoctoral Research Assistant who works in Soft and Biological Matter at the Rudolf Peierls Centre for Theoretical Physics, Department of Physics, Oxford University, said:
“The ability to get even a tiny amount of mechanical work from these biological systems is valuable because they do not need an input power and use internal biochemical processes to move around.”
“At micro scales, our simulations show that the flow generated by biological assemblies is capable of reorganising itself in such a way as to generate a persistent mechanical power for rotating an array of microrotors.”

Senior author Prof. Julia Yeomans, also from the Rudolf Peierls Centre for Theoretical Physics at Oxford, added:
“Nature is brilliant at creating tiny engines, and there is enormous potential if we can understand how to exploit similar designs.”
In an Abstract preceding the main paper in the journal, the scientists wrote:
“The lattice of rotors self-organizes into a spin state where neighboring discs continuously rotate in permanent alternating directions due to combined hydrodynamic and elastic effects.”
“Our virtual prototype demonstrates a new research direction for the design of micromachines powered by the nematohydrodynamic properties of active turbulence.”
Citation: “Active micromachines: Microfluidics powered by mesoscale turbulence,” Sumesh P. Thampi, Amin Doostmohammadi, Tyler N. Shendruk, Ramin Golestanian and Julia M. Yeomans. Science Advances, Vol. 2, no. 7, e1501854. 8th July, 2016. DOI: 10.1126/sciadv.1501854.
