Nuclear fission is where the unstable nucleus of a heavy atom splits either spontaneously or after absorbing a nuclear particle.
In both cases the reaction releases a lot of energy and results in two smaller nuclei and other nuclear particles.
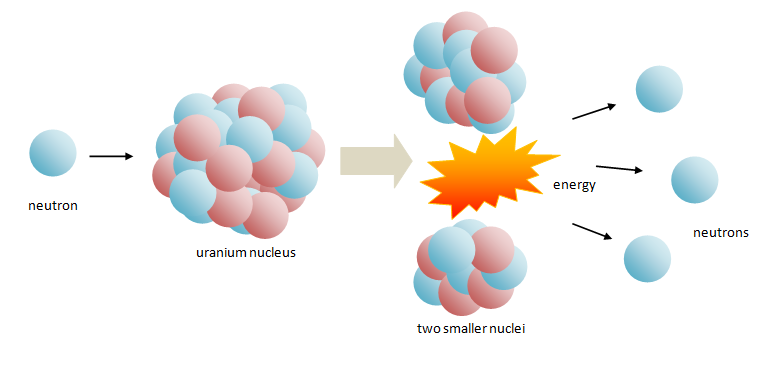
A commonly described nuclear fission reaction is that of uranium breaking up after absorbing a neutron.
When a neutron hits a uranium nucleus the nucleus absorbs it. But the extra neutron is enough to cause the uranium nucleus to become unstable – so it splits into two smaller nuclei.
Example of nuclear fission
Below is an illustration of nuclear fission occurring:
Nuclear fission can turn into a chain reaction
A nuclear fission reaction releases a lot of energy and also throws out three more neutrons, which may then collide with more uranium nuclei.
If every neutron released this way collides with another uranium nucleus, the result is a rapidly expanding chain reaction that can lead to a huge explosion – the basis of a nuclear bomb. This only happens under certain ‘critical mass’ conditions that concentrate enough unstable uranium in a small space.
It is also possible to create a chain reaction but hold it within limits, resulting in a controlled release of energy – this is the basis of a nuclear reactor.
Another element that is also used in nuclear reactors is plutonium. The principle of fission using plutonium is the same as for uranium: a neutron collides with a heavy nucleus, causing it to break up into two lighter nuclei and more neutrons, and releasing a lot of energy.
Where does the energy come from?
There is a lot of energy in the bonds that hold the particles of the nucleus together. These bonds are much stronger for instance than the bonds that hold electrons in place in atoms and molecules.
Nuclear fission is an example of an exothermic reaction – energy is released because the nuclei of the end products of fission are more tightly bound than the original uranium nucleus.
This release of energy coincides with a ‘mass deficit’ in that the total mass of the lighter nuclei and particles after the reaction is a tiny amount less than the mass of the heavy nucleus and neutron before the reaction.
The lost mass is converted to energy according to Einstein’s famous e=mc² equation. Thus even though m is a very small mass, when multiplied by the square of the very large number c (the speed of light), the amount of energy released e, is huge.
The energy that is released in a fission reaction that splits one uranium atom is about 200 MeV (mega electron volts), which is millions of times more than the amount of energy released in burning an atom of carbon.
This is why nuclear fuel is a much more concentrated source of energy than fossil fuel.
The atomic bomb that devastated Hiroshima in 1945 consumed just 0.6 grams of uranium.
This article forms part of a series that includes:
- What is nuclear fusion?
- What is nuclear energy?
- The difference between nuclear fission and nuclear fusion