
Lithium-ion batteries are currently the most important battery technology. However, they are expensive and contain a flammable liquid – making them hazardous.
Solid-state sodium-ion batteries are safer than lithium-ion batteries, but their performance hasn’t been good enough to offset the safety advantages. Lithium-ion batteries use an organic solvent which is flammable and they have a relatively short useful life. Solid-state batteries, on the other hand, use both solid electrodes and solid electrolytes.
Researchers from the University of Houston found that using an organic cathode dramatically improves both stability and energy density. The team reported, for the first time, a reversible active material-electrolyte interfacial resistance evolution during cycling.
The findings were published in the journal Joule (citation below).
The organic cathode material, pyrene-4,5,9,10-tetraone (PTO), enabled high-performance in sulfide-based all-solid-state sodium batteries (ASSSBs).
According to the summary of the study, “the PTO-based cells exhibit a high specific energy (587 Wh kg −1) and a record cycling stability (500 cycles) among ASSSBs.”
“This work reveals an effective cathode material design strategy toward compatibility with solid electrolytes and thus high-performance ASSSBs,” the summary added.
Yan Yao, associate professor of electrical and computer engineering at the University of Houston and corresponding author of the paper, said:
“We found for the first time that the resistive interface that forms between the cathode and the electrolyte can be reversed. That can contribute to stability and longer cycle life.”
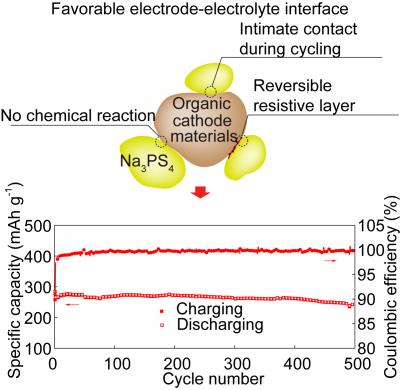
A low-modulus cathode material, such as PTO, maintains the intimate interfacial contact with solid electrolytes during cycling, thus improving cycle life.
According to Yanliang “Leonard” Liang, a research assistant professor in the UH Department of Electrical and Computer Engineering, the reversibility of the interface is the key, allowing the solid-state battery to reach a higher energy density without affecting cycle life.
Normally, a solid-state battery’s ability to store energy is halted when the resistive cathode electrolyte interface forms. But reversing that resistance allows energy density to remain high during cycling, Liang said.
Finding a solid electrolyte with conductivity matching that of the liquid electrolytes used in lithium-ion batteries was a major challenge. But now that there are sufficiently conductive solid electrolytes are available, a new challenge has been attaining stable active material-solid electrolyte interfaces.
Fang Hao, a PhD student working in Yao’s group, said the organic cathode is more pliable and thus able to remain in contact with the interface, improving cycling life.
“If you have reliable contact between the electrode and electrolyte, you will have a great chance of creating a high-performance solid-state battery,” Hao said.
Citation
Fang Hao, Xiaowei Chi, Yanliang Liang, Kejie Zhao, Jun Lou, Yan Yao, “Taming Active Material-Solid Electrolyte Interfaces with Organic Cathode for All-Solid-State Batteries,” Joule, April 19, 2019, DOI:https://doi.org/10.1016/j.joule.2019.03.017

