Water can now be obtained from the air using only sunlight – a scientific breakthrough that will completely transform the current quality of life of billions of people across the planet who face serious shortages. The scientists, from Massachusetts Institute of Technology (MIT) and the University of California, Berkeley, say they have an appliance that literally pulls all a household’s water needs from the air, even in arid deserts, using only the power of the Sun.
Omar M. Yaghi, Professor of Chemistry, UC Berkeley, and colleagues believe that the future is just around the corner, after they demonstrated a harvester that uses only the light from the Sun to pull several liters of water from the air each day in conditions as dry as 20% humidity – a level typically found in arid areas.
Prof. Yaghi and team members explained in the journal Science that their solar-powered harvester uses a special material – MOF (Metal-Organic Framework) – that scientists produced at UC Berkeley.
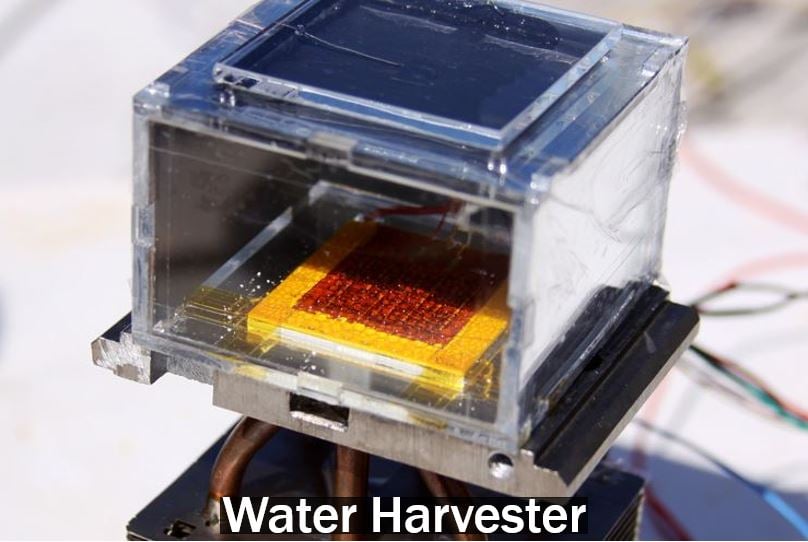 Even in desert-like conditions, this harvester can obtain enough water to satisfy the requirements of an average household. Imagine how it could transform the lives of people who have to survive in areas with virtually endless droughts. (Image: UC Berkeley)
Even in desert-like conditions, this harvester can obtain enough water to satisfy the requirements of an average household. Imagine how it could transform the lives of people who have to survive in areas with virtually endless droughts. (Image: UC Berkeley)
Water-harvesting device – a major breakthrough
Prof. Yaghi said:
“This is a major breakthrough in the long-standing challenge of harvesting water from the air at low humidity. There is no other way to do that right now, except by using extra energy. Your electric dehumidifier at home ‘produces’ very expensive water.”
Over a 12-hour period, using 2.2 pounds (1 kg) of MOF, the prototype was able to pull 3 quarts (2.8 liters) of water from thin air in a twenty-to-thirty percent humidity environment.
MIT carried out rooftop tests which confirmed that the device works successfully in real-world conditions.
Prof. Yaghi added:
“One vision for the future is to have water off-grid, where you have a device at home running on ambient solar for delivering water that satisfies the needs of a household. To me, that will be made possible because of this experiment. I call it personalized water.”
 The device pulls 2.8 liters of water out of the atmosphere per day for each kilogram of MOF it contains. (Image: Adapted from Science journal picture)
The device pulls 2.8 liters of water out of the atmosphere per day for each kilogram of MOF it contains. (Image: Adapted from Science journal picture)
MOFs have been around since 1990s
The MOFs were invented by Prof. Yaghi over two decades ago, combining organic molecules and metals like aluminum and magnesium in a tinker-toy arrangement to create extremely rigid, porous structures which are ideal for storing liquids and gases.
Over the past twenty years, over 20,000 different MOFs have been created by scientists globally. Some of them hold methane, hydrogen, and other chemicals.
German chemical giant BASF is testing one MOF created by Prof. Yaghi in natural gas-fueled trucks. MOF tanks can hold three times the methane that can currently be pumped under pressure into an empty fuel tank.
Some MOFs can capture carbon dioxide (CO2) from flue gases, separate petrochemicals within processing plants, or catalyze the reactions of absorbed chemicals.
2014 – MIT and UC Berkeley join forces
Three years ago, Yaghi and his team at UC Berkeley synthesized a MOF – a combination of adipic acid and zirconium metal – that binds water vapor. He suggested to Evelyn Wang, Associate Professor in Mechanical Engineering at MIT, that they join forces to create a water-collecting system using his MOF.
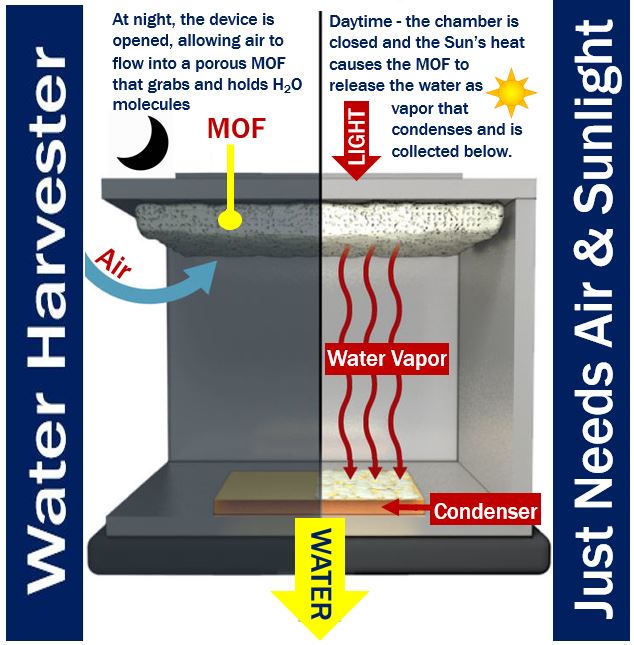
Prof. Wang and her team members designed a system consisting of over two pounds (about 1 kg) of dust-sized MOF crystals compressed between a condenser plate and a solar absorber – this was placed inside a chamber open to the air.
 Crystalline materials similar to the ones in this image can harvest water vapor from air. (Image: Science Journal)
Crystalline materials similar to the ones in this image can harvest water vapor from air. (Image: Science Journal)
H2O molecules preferentially attach to the interior surfaces as ambient air diffuses through the porous MOF. According to X-ray diffraction studies, the water vapor molecules tend to gather in groups of eight to form cubes.
Sunlight passes through a window, heats up the MOF, and drives the bound water into a condenser, which is at ambient temperature. The condensing water vapor drips into a collector.
Prof. Wang said:
“This work offers a new way to harvest water from air that does not require high relative humidity conditions and is much more energy efficient than other existing technologies.”
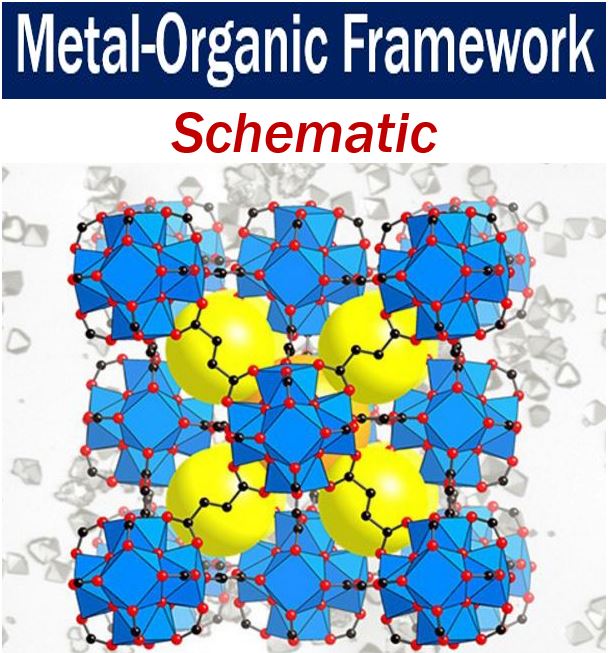 The lines in this schematic of a MOF are organic linkers – the intersections are metal ions. They are the building blocks that Prof. Yaghi stitches together into a crystalline sponge-like material using reticular chemistry. The porous spaces that can be filled with liquid or gas represented by the yellow balls above. (Image: UC Berkeley)
The lines in this schematic of a MOF are organic linkers – the intersections are metal ions. They are the building blocks that Prof. Yaghi stitches together into a crystalline sponge-like material using reticular chemistry. The porous spaces that can be filled with liquid or gas represented by the yellow balls above. (Image: UC Berkeley)
Further research needed
Further studies are required to improve the proof-of-concept harvester, the authors say. Currently, their MOF can only absorb 20% of its weight in water. With other MOF materials, they believe it would be able to absorb at least 40% of its weight in water.
They also want to tweak the material so that it is more effective at lower and higher humidity levels.
Prof. Yaghi said:
“It’s not just that we made a passive device that sits there collecting water; we have now laid both the experimental and theoretical foundations so that we can screen other MOFs, thousands of which could be made, to find even better materials.”
“There is a lot of potential for scaling up the amount of water that is being harvested. It is just a matter of further engineering now.”
 Professors Yaghi and Wang. (Images: 1. Left – UC Berkeley. 2. Right – MIT)
Professors Yaghi and Wang. (Images: 1. Left – UC Berkeley. 2. Right – MIT)
Prof. Wang and her team are currently continuing to improve the water harvesting system, while Prof. Yaghi and colleagues are working at making the MOFs more effective.
Prof. Yaghi said:
“To have water running all the time, you could design a system that absorbs the humidity during the night and evolves it during the day. Or design the solar collector to allow for this at a much faster rate, where more air is pushed in.”
“We wanted to demonstrate that if you are cut off somewhere in the desert, you could survive because of this device. A person needs about a Coke can of water per day. That is something one could collect in less than an hour with this system.”
In an article published in the journal Science, Robert Service wrote that there are approximately 13 trillion liters of water floating around in the atmosphere at any one time. This is equivalent to ten percent of all the freshwater in our planet’s rivers and lakes.
Last week, scientists from the University of Manchester in England announced the development of a scalable graphene membrane that can filter out the salt form seawater, making it drinkable.
Video – pulling water from thin air
In this UC Berkely video, Prof. Yaghi explains how this new device works and how it could transform the lives of billions of people across the planet who have to survive in areas that are becoming progressively more arid.
