Using 3D printing, researchers can now predict how leaky heart valves are. They created a new integrated workflow that improves valve sizing accuracy during aortic valve replacement procedures.
The researchers created a new 3D printing workflow that allows specialists to evaluate how different valve dimensions will interact with each individual patient’s unique anatomy.
This protocol utilizes scan data to create physical models of a patient’s aortic valve, as well as a ‘sizer’ device to determine the exact size of the replacement valve.
The researchers wrote about their work in the Journal of Cardiovascular Computed Tomography (citation below). The authors were Ahmed Hosny, Joshua D. Dilley, Tatiana Kelil, Moses Mathur, Mason N. Deane, James C. Weaver, and Beth Ripley. They are from the Harvard University’s Wyss Institute for Biologically Inspired Engineering, Massachusetts General Hospital, Brigham and Women’s Hospital, The University of Washington, and the Max Planck Institute of Colloids and Interfaces.
System accurately-sizes heart valves
Corresponding author, James Weaver, Ph.D., a Senior Research Scientist at the Wyss Institute, said:
“If you buy a pair of shoes online without trying them on first, there’s a good chance they’re not going to fit properly.”
“Sizing replacement TAVR valves poses a similar problem, in that doctors don’t get the opportunity to evaluate how a specific valve size will fit with a patient’s anatomy before surgery.”
“Our integrative 3D printing and valve sizing system provides a customized report of every patient’s unique aortic valve shape, removing a lot of the guesswork and helping each patient receive a more accurately sized valve.”
TAVR stands for transcatheter aortic valve replacement.

CT scans do not show up parts of heart valves clearly
Patients who need a replacement heart valve typically get a CT scan. CT scans take a series of X-ray images of the heart. They subsequently create a 3D reconstruction of the heart’s internal anatomy. With the CT scan, specialists get a good look at the wall of the aorta. They can also see any associated calcified deposits.
However, the delicate ‘leaflets’ of tissue that close and open the valve are usually not thick enough to be clearly visible.
Dr. Weaver explained:
“After a 3D reconstruction of the heart anatomy is performed, it often looks like the calcified deposits are simply floating around inside the valve, providing little or no insight as to how a deployed TAVR valve would interact with them.”
Dr. Weaver explained:
“After a 3D reconstruction of the heart anatomy is performed, it often looks like the calcified deposits are simply floating around inside the valve, providing little or no insight as to how a deployed TAVR valve would interact with them.”
Special computer program
Ahmed Hosny, PhD Applicant at Harvard Medical School’s Department of Radiation Oncology, created a computer program that uses parametric modeling to generate virtual 3D models of the valve’s leaflets.
The software uses seven coordinates of each individual patient’s valve that show up on the CT scans. He then merged the digital leaflet models with the CT data and adjusted so that they fit into the heart valve correctly.
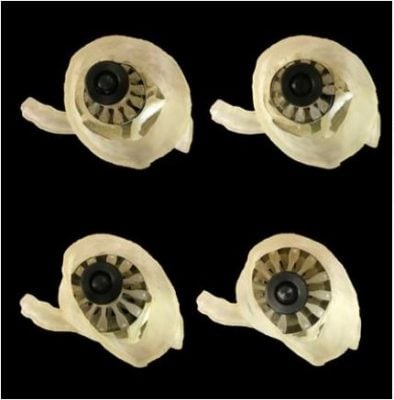
The resulting model incorporated the leaflets with their associated calcified deposits. It was then 3D printed into a physical, three-dimensional multi-material model.
The researchers also 3D printed a custom ‘sizer‘ device which fits inside the valve model that had been 3D printed. The device grows and shrinks to determine the exact size of the artificial valve for each specific patients.
They then wrapped a thin layer of pressure-sensitive film around the sizer to map the pressure between the 3D printed heart valves, their associated calcified deposits, and the sizer, while gradually expanding the sizer.
According to a press release by the Wyss Institute for Biologically Inspired Engineering:
“In addition, the multi-material design of the 3D-printed valve models, which incorporate flexible leaflets and rigid calcified deposits into a fully integrated shape, could much more accurately mimic the behavior of real heart valves during artificial valve deployment, as well as provide haptic feedback as the sizer is expanded.”
Testing the system
The researchers tested their system against data from thirty patients. They had all already undergone transcatheter aortic valve replacement procedures. Fifteen of them had developed leaks from heart valves because they were too small.
The team predicted what size valve each patient should have received. They also predicted whether the patients would experience post-procedure leaks. They based this on how well the sizer fit into the 3D printed aortic valve models.
The system accurately predicted leak outcome in between 60% and 73% of the patients, depending on the type of valve they had received. It also determined that sixty percent of them had received a valve of the right size.
Predicting likelihood of complications from TAVR critical
Co-author Beth Ripley, M.D., Ph.D, an Assistant Professor in the Department of Radiology at the University of Washington, said:
“Being able to identify intermediate- and low-risk patients whose heart valve anatomy gives them a higher probability of complications from TAVR is critical, and we’ve never had a non-invasive way to accurately determine that before.”
“Those patients might be better served by surgery, as the risks of an imperfect TAVR result might outweigh its benefits. Additionally, being able to physically simulate the procedure might inform future iterations of valve designs and deployment approaches.”
Prof. Ripley was a Cardiovascular Imaging Fellow at Brigham and Women’s Hospital during the study.
All freely available online
The team’s leaflet modeling software and 3D printing software protocol is freely available online. Any clinician and researcher who wants to use them can.
The authors hope that their project becomes a springboard for evolvable biomedical design that keeps up with the market’s state of the art.
Founding Director of the Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., said:
“At the core of the personalized medicine challenge is the realization that one medical treatment will not serve all patients equally well, and that therapies should be tailored to the individual.”
“This principle applies to medical devices as well as drugs, and it is exciting to see how our community is innovating in this space and attempting to translate new personalized approaches from the lab and into the clinic.”
Citation
“Pre-procedural fit-testing of TAVR valves using parametric modeling and 3D printing,” Ahmed Hosny, Joshua D. Dilley, Tatiana Kelil, Moses Mathur, Mason N. Deane, James C. Weaver, and Beth Ripley. Journal of Cardiovascular Computed Tomography (2018). DOI: https://doi.org/10.1016/j.jcct.2018.09.007.

