A team of American scientists has discovered on Earth how dense, alien ice can transform from water within just a few billionths of a second. For the first time, researchers have captured the freezing of water, molecule-by-molecule, into a weird, dense form known as Ice VII (pronounced ‘ice seven’). Ice VII is a common type of alien ice that is found naturally in outer space, for example, when icy planetary bodies collide.
In addition to helping researchers gain a better understanding of distant worlds, the team of scientists explained in an article published in Physical Review Letters on July 11th, titled – “Compression Freezing Kinetics of Water to Ice VII” – that their findings could reveal how water and other elements and compounds undergo transitions from liquids to solids.
If we can learn how to manipulate those transitions, we might one day be able to engineer materials with exotic new properties, the authors, from Stanford University, Los Alamos National Laboratory, SLAC National Accelerator Laboratory, Sandia National Laboratories, and Lawrence Livermore National Laboratory, explained.
Study leader Arianna Gleason, a postdoctoral fellow at Los Alamos National Laboratory, and also a visiting scientist in the Extreme Environments Laboratory of the School of Earth, Energy & Environmental Sciences at Stanford, said:
“These experiments with water are the first of their kind, allowing us to witness a fundamental disorder-to-order transition in one of the most abundant molecules in the universe.”
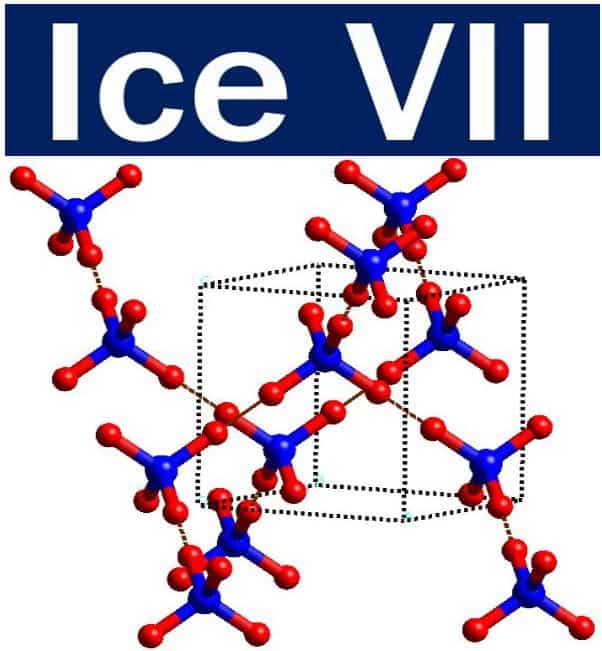 The crystal structure of Ice VII, a common type of alien ice. Ice VII is a cubic crystalline form of ice which can be formed from liquid water above 30,000 atmospheres by lowering its temperature to room temperature. Researchers hypothesize that Ice VII may comprise the ocean floor of Titan, Saturn’s largest moon. (Image: adapted from Wikipedia)
The crystal structure of Ice VII, a common type of alien ice. Ice VII is a cubic crystalline form of ice which can be formed from liquid water above 30,000 atmospheres by lowering its temperature to room temperature. Researchers hypothesize that Ice VII may comprise the ocean floor of Titan, Saturn’s largest moon. (Image: adapted from Wikipedia)
Phase changes happen in billionths of a second
Scientists have been studying how substances undergo phase changes between solid, liquid and gas states for a long time. Phase changes can sometimes occur on the minuscule scale of mere atoms, and at incredibly fast speeds.
Researchers have struggled to capture the moment-to-moment action of phase changes, and have worked backwards, starting with stable solids when trying to piece together the molecular steps that predecessor liquids took.
Senior author, Wendy Mao, associate professor of geological sciences, and a principal Stanford investigator, said:
“There have been a tremendous number of studies on ice because everyone wants to understand its behavior. What our new study demonstrates, and which hasn’t been done before, is the ability to see the ice structure form in real time.”
 Did you know that there is lots of ice on the surface of Mars? (Image: adapted from astropedia.astrogeology.usgs.gov)
Did you know that there is lots of ice on the surface of Mars? (Image: adapted from astropedia.astrogeology.usgs.gov)
X-Ray laser caught alien ice in the act
In this latest research, those timescales became achievable thanks to the world’s most powerful X-ray laser – the Linac Coherent Light Source – located at the nearby SLAC National Accelerator Laboratory.
There, the scientists beamed an intense, green-colored laser at a tiny target containing a sample of liquid water. The Linac Coherent Light Source instantly vaporized layers of a diamond on one side of the target, generating a rocket-like force which compressed the water to pressures in excess of 50,000 Earth sea-level atmospheres.
As the water compacted, another beam from an X-ray Free Electron Laser (an instrument) arrived in a series of ultra-short bright pulses. Each pulse was just one femtosecond, i.e. one quadrillionth of a second long (1/1,000,000,000,000 of a second).
Like a series of camera flashes, this strobing X-ray laser rapidly snapped several images revealing the progression of molecular changes, flip book-style, while the water under pressure crystallized into Ice VII, a common type of alien ice.
The phase took six-billionths of a second, i.e. just nanoseconds. The scientists were surprised to discover that the water molecules bonded into rod shapes, and not the spheres that theory had predicted.
According to an article published in Eurekalert.org:
“The platform developed for this study – combining high pressure with snapshot images – could help researchers probe the myriad ways water freezes, depending on pressure and temperature.”
“Under the conditions on our planet’s surface, water crystallizes in only one way, dubbed ice Ih (‘ice one-H’) or simply “hexagonal ice,” whether in glaciers or ice cube trays in the freezer.”
The more scientists know about alien ice types, including Ice VII, the better they will be at modeling such remote environments as comet impacts, the internal structures of water-filled moons such as Jupiter’s Europa, which might support life as we know it, and super-Earths – jumbo, rocky, oceanic **exoplanets.
** An exoplanet is a planet that orbits a star outside our solar system.
Gleason said:
“Any icy satellite or planetary interior is intimately connected to the object’s surface. Learning about these icy interiors will help us understand how the worlds in our solar system formed and how at least one of them, so far as we know, came to have all the necessary characteristics for life.”
In an Abstract preceding the main article in the journal, the authors wrote:
“These first XRD data demonstrate rapid growth kinetics of ice VII with implications for fundamental physics of diffusion-mediated crystallization and thermodynamic modeling of collision or impact events on ice-rich planetary bodies.”
The study was funded by the Los Alamos National Laboratory, the National Science Foundation, the US Department of Energy Office of Science, the SLAC National Accelerator Laboratory, and Fusion Energy Science.
Video – Ice VII and other solid forms of water
Did you know that there are at least seventeen solid forms of water, i.e. seventeen types of ice?
