Sustainable energy sources, such as wind and solar power, are essential for the future of our planet. However, they present a significant challenge: they don’t always produce electricity when we need it most.
To address this, it is crucial to develop cost-effective and efficient methods to store the energy they generate, ensuring a steady power supply even when the wind isn’t blowing or the sun isn’t shining.
Researchers at Columbia Engineering have been working on creating innovative battery systems that can improve the storage of renewable energy.
In a recent study published on September 5 in Nature Communications (citation below), the team utilized a combination of potassium (K), sodium (Na), and sulfur (S) to develop K-Na/S batteries.
These materials, which are both inexpensive and widely available, offer a promising solution for low-cost, long-term energy storage.
Yuan Yang, an associate professor of materials science and engineering at Columbia Engineering, explained:
“It’s essential that we extend the lifespan of these batteries and make them easy and affordable to produce.” Yang emphasizes that improving renewable energy storage will enhance grid stability, lessen reliance on fossil fuels, and contribute to a more sustainable energy landscape.
Innovative Electrolyte Improves Battery Efficiency
K-Na/S batteries face two significant challenges:
- They tend to have a low energy capacity due to the formation of solid K2S2 and K2S, which hinders the energy diffusion process.
- They need extremely high operating temperatures, above 250°C, which adds complexity and cost.
Previous research efforts have struggled to resolve these issues, but Yang’s team has taken a new approach to enhance battery performance.
-
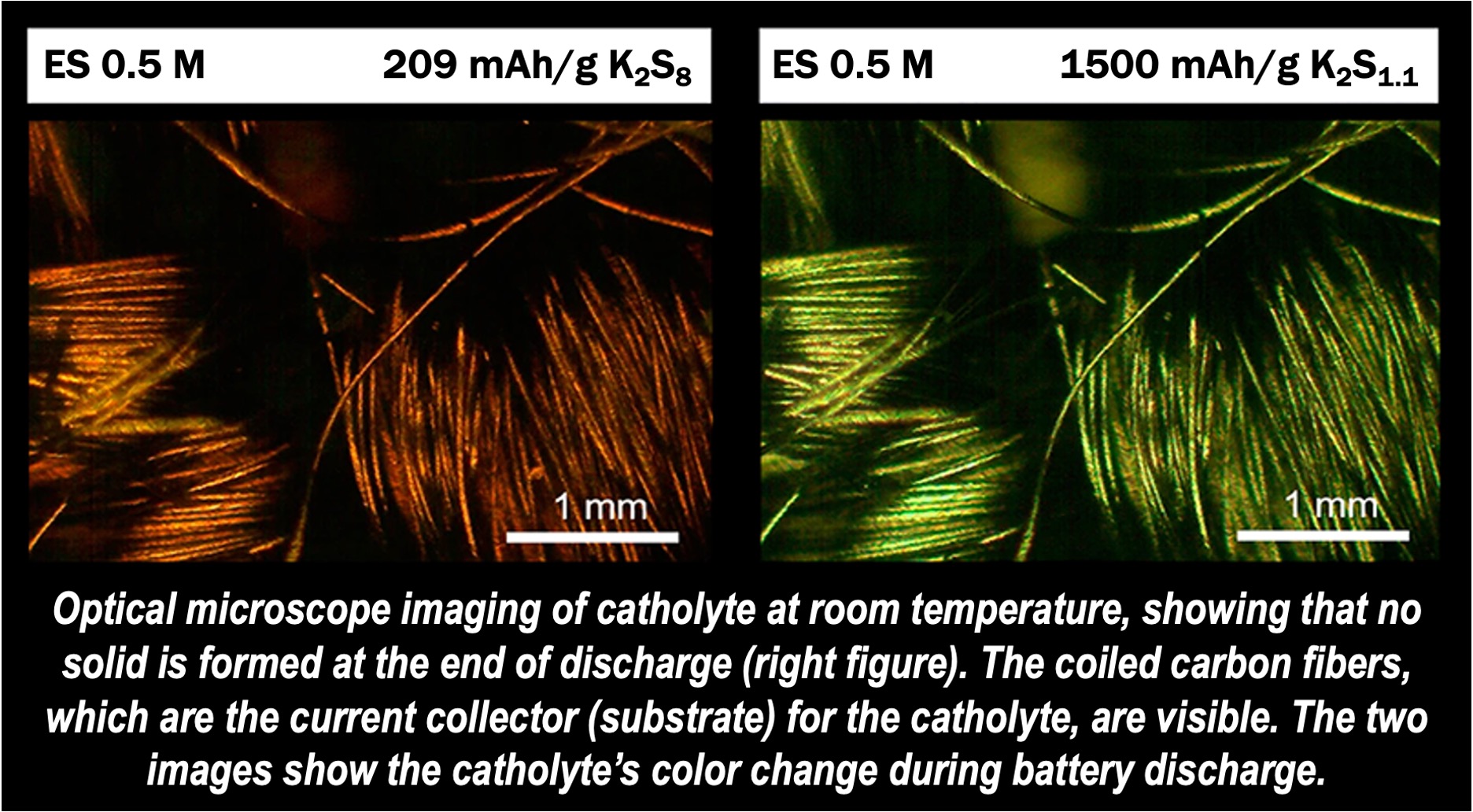
Electrolyte solution improves energy density
The team created a novel electrolyte solution, composed of acetamide and ε-caprolactam, which can dissolve the problematic solid compounds, improving the battery’s energy density.
This solution also allows the battery to function at much lower temperatures—about 75°C—without sacrificing energy capacity, making it far more practical and affordable than earlier designs.
Zhenghao Yang, one of the lead authors and a PhD student working under Yuan Yang, said:
“We’re achieving nearly maximum discharge capacities and significantly increasing the cycle life of these batteries, which is a major step forward for intermediate-temperature K/S batteries.”
Paving the Way for a Sustainable Energy Future
Yang’s group is part of the Columbia Electrochemical Energy Center (CEEC), which brings together experts from across Columbia’s School of Engineering and Applied Science.
The CEEC collaborates with various industries to drive innovation in electrochemical energy storage and conversion, helping to accelerate the development and commercial application of new technologies.
Looking Toward Large-Scale Implementation
Currently, the researchers are focusing on small, coin-sized battery prototypes, but their ultimate goal is to scale this technology to handle large-scale energy storage needs.
If successful, these advanced batteries could offer a reliable power supply from renewable energy sources, even during periods of limited sunlight or wind.
The team is now refining the electrolyte composition as they work toward this larger vision.
Citation
Tian, L., Yang, Z., Yuan, S. et al. Designing electrolytes with high solubility of sulfides/disulfides for high-energy-density and low-cost K-Na/S batteries. Nat Commun 15, 7771 (2024). https://doi.org/10.1038/s41467-024-51905-6
