A new battery could make use of carbon dioxide, a greenhouse gas, before it ever gets into the atmosphere. Researchers from MIT say we could make a lithium-based battery partly from carbon dioxide in power plants.
Carbon dioxide or CO2 is a greenhouse gas. A greenhouse gas is an atmospheric gas that absorbs and emits radiation. In other words, it keeps our planet’s surface warm. There is a worldwide effort to reduce our emissions of greenhouse gases.
New battery converts CO2
Rather than using metal catalysts to convert carbon dioxide into specialized chemicals, this new battery could continuously convert it. As it discharges, the battery could convert the CO2 into a solid carbonate.
Aliza Khurram, Mingfu He, and Betar M. Gallant wrote about their research in the journal Joule (citation below).
Gallant is an Assistant Professor of Mechanical Engineering. Khurram is a doctoral student and He a postdoc. The three researchers are from MIT (Massachusetts Institute of Technology).
The authors say that their research is still at an early stage. In other words, their new battery is far from commercial deployment. However, it could eventually open up new avenues for tailoring CO2 conversion reactions. This could ultimately help reduce our greenhouse gas emissions.
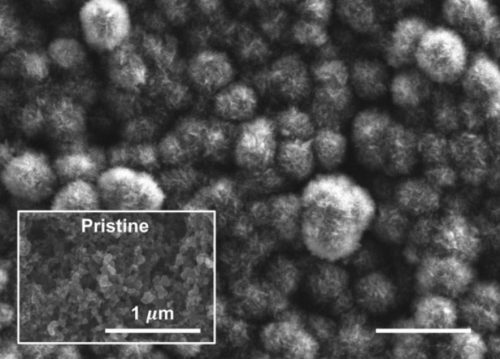
The new battery consists of carbon, lithium metal, and an electrolyte that the scientists designed.
Changing the economics of power plants
Power plants today use up to thirty percent of the electricity they generate capturing CO2. Anything that can help reduce the cost of that capture has value. Anything that can result in an end product also has value.
Carbon dioxide, however, is not very reactive. Therefore, attempting to find new reaction pathways is important, Prof. Gallant explains. Generally, the only way to get CO2 to become reactive under electrochemical conditions is with very high voltage inputs. However, apart from being inefficient, these processes are also expensive.
Ideally, carbon dioxide would undergo reactions that produce something we could use. A fuel, for example, is something we could use. A chemical is also something that could be useful.
Efforts at electrochemical conversion, however, which scientists usually conduct in water, remain hindered by the poor selectivity of chemicals produced and high energy inputs.
CO2-loaded electrolytes
The researchers looked into whether they could use carbon-dioxide-capture chemistry to make CO2-loaded electrolytes. These electrolytes are one of three vital parts of a battery. The captured gas would subsequently be used during the battery’s discharge to provide a power output.
Gas capture sequestration (CCS) releases the CO2 back to the gas phase for long-term storage. Most CCS techniques capture the power plant’s carbon dioxide through a chemical absorption process. The process then either chemically alters it into a fuel or a chemical feedstock. It is also possible to store the carbon dioxide underground.
This team developed a completely new approach. They wanted to make material for a new battery’s component right from the power plant’s waste stream.
There has been growing interest in the development of lithium-carbon-dioxide batteries. They use CO2 as a reactant during discharge. It has been necessary to use metal catalysts because of carbon dioxide’s low reactivity.
Not only are these catalysts expensive, but their function remains poorly understood. Also, it is difficult to control the reactions.
Incorporating CO2 in a liquid state
The researchers found a way to achieve electrochemical CO2 conversion using just a carbon electrode. They incorporated the carbon dioxide in a liquid state. The key is to preactivate the CO2 by incorporating the gas into an amine solution.
Prof. Gallant said:
“What we’ve shown for the first time is that this technique activates the carbon dioxide for more facile electrochemistry.”
“These two chemistries – aqueous amines and nonaqueous battery electrolytes – are not normally used together, but we found that their combination imparts new and interesting behaviors that can increase the discharge voltage and allow for sustained conversion of carbon dioxide.”
Through a series of experiments, the researchers showed that their approach does work. It can produce a new battery. Specifically, a new lithium-carbon dioxide battery. Additionally, its voltage and capacity are competitive with that of state-of-the-art lithium-gas batteries.
Moreover, the amine, which acts as a molecular promoter, is not consumed in the reaction.
They used a nonaqueous electrolyte
According to Khurram, the key was developing the right electrolyte system. The researchers decided to use a nonaqueous electrolyte in this initial proof-of-concept study.
A nonaqueous electrolyte would limit the available reaction pathways, the authors explained. It would, therefore, make it easier to characterize the reaction and determine how viable it was.
The researchers chose an amine that CCS applications currently use. However, nobody had applied it to batteries.
According to an MIT press release:
“This early system has not yet been optimized and will require further development, the researchers say.”
“For one thing, the cycle life of the battery is limited to 10 charge-discharge cycles, so more research is needed to improve rechargeability and prevent degradation of the cell components.”
A new battery is years away
Prof. Gallant says that a new battery, specifically a lithium-carbon dioxide battery, is years away as a viable product.
According to Prof. Gallant, their research covers just one of the many advances we need to make.
The concept, however, offers great potential, according to Prof. Gallant. Carbon capture is essential to meeting global goals for reducing greenhouse gas emissions. There are not yet, however, proven, long-term ways of disposing of carbon dioxide. Also, there are no proven, long-term ways of using all the resulting CO2.
The leading contender is still underground geological disposal. However, this approach remains somewhat unproven. Nobody knows how much it can accommodate. Additionally, it requires extra energy for pumping and drilling.
The research team is also looking into the possibility of developing a continuous-operation version of the process.
It would use a steady stream of CO2 under pressure with the amine material. In other words, there would not be a preloaded supply of the material. It would thus allow it to deliver a steady power output. A steady power output that is, as long as the new battery gets its supply of carbon dioxide.
An integrated system one day
Ultimately, the authors hope to make this into an integrated system. The system would carry out the capture of CO2 from the power plant’s emissions stream. It would also convert it into an electrochemical material which could then be used in batteries.
Prof. Gallant says:
“It’s one way to sequester it as a useful product.”
Citation
“Tailoring the Discharge Reaction in Li-CO2 Batteries through Incorporation of CO2 Capture Chemistry,” Aliza Khurram, Mingfu He, and Betar M. Gallant. Joule. Published: September 21, 2018. https://doi.org/10.1016/j.joule.2018.09.002.
