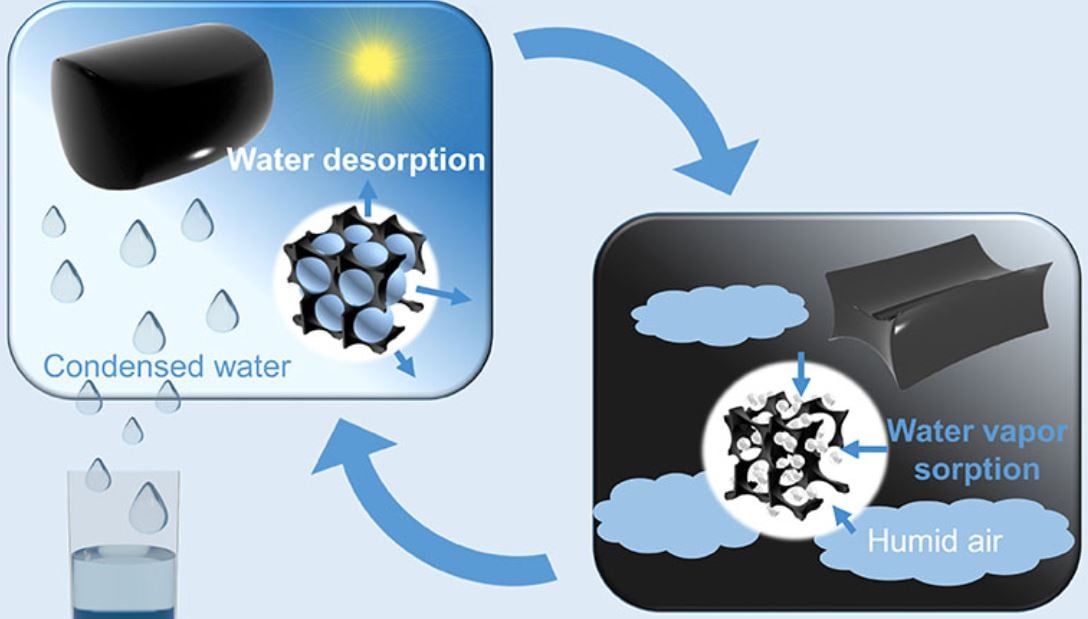
Researchers at KAUST in Saudi Arabia have developed a device that sucks drinking water from dry desert air. KAUST stands for King Abdulla University of Science and Technology. The simple device can capture its own weight in water from the night air. It then releases the water when sunlight warms it.
The researchers say that their new device could provide a secure and reliable new source of drinking water in remote arid regions.
Drinking water in our atmosphere
Our atmosphere contains a lot of water; about nearly 13 trillion tons of it. It is a massive renewable reservoir of clean, potable (drinking) water.
There have been many research projects globally in which scientists tested materials and devices to tap this water source. However, most of them have either been too expensive, inefficient, or complex for practical use.
Peng Wang and colleagues have developed a promising prototype device. Wang is an Associate Professor at the Water Desalination and Reuse Center at KAUST’s Division of Biological and Environmental Science and Engineering.
Prof. Wang and colleagues wrote about their work in the journal Environmental Science & Technology (citation below). The other authors, all from KAUST, were Renyuan Li, Yusuf Shi, Mossab Alsaedi, Mengchun Wu, and Le Shi.
About the drinking water device
Calcium chloride, a cheap, stable, non-toxic salt, is at the heart of the device. Calcium chloride is a deliquescent salt, i.e., it absorbs moisture from the air and dissolves in it. It absorbs so much vapor from the atmosphere that eventually a pool of liquid forms.
Renyuan Li said:
“The deliquescent salt can dissolve itself by absorbing moisture from air.”
Li is a Ph.D. Student at KAUST. His interests include the rational design of surfaces and the micro/nano-fluidics of water and energy applications.

The problem with calcium chloride
Calcium chloride has great potential in the harvesting of water. However, it turns from a solid into a salty liquid after absorbing water. Salt water is not drinking water. This has been a major obstacle for its use in water capture devices. “Systems that use liquid sorbents are very complicated,” Li explained.
To overcome this problem, the scientists incorporated the salt into a hydrogel (polymer). This hydrogel could not only hold lots of water, but it also remained in a solid state.
Additionally, the researchers added a tiny amount of carbon nanotubes (0.42% by weight). This ensured the release of the captured water vapor.
Carbon nanotubes absorb sunlight efficiently and then convert that captured energy into heat.
The researchers incorporated thirty-five grams of this material into a simple prototype device. They then left it outside overnight. By next morning, it had captured 37 grams of water. The relative humidity of the night air was approximately 60%.
After the sun had risen, following 2.5 hours of natural sunlight irradiation, most of the sorbed water was released and collected inside the prototype device.
Cheap drinking water
Li said:
“The hydrogel’s most notable aspects are its high performance and low cost.”
If the researchers can scale up their prototype to produce three liters of water per day, the material cost of the absorbent hydrogel would be just half-a-cent per day. An average adult requires at least three liters of drinking water per day.
The authors plan to fine tune the absorbent hydrogel. They want it to release harvested drinking water continuously, rather than in batches, Prof. Wang explained.
In 2017 in a different project, scientists from the University of Manchester turned seawater into drinking water using a scalable graphene membrane.
Citation
“Hybrid Hydrogel with High Water Vapor Harvesting Capacity for Deployable Solar-Driven Atmospheric Water Generator,” Renyuan Li, Yusuf Shi, Mossab Alsaedi, Mengchun Wu, Le Shi, and Peng Wang. Environmental Science & Technology. 2018, 52 (19), pp 11367–11377. DOI: 10.1021/acs.est.8b02852.