What is galvanized steel?
We get galvanized steel if we coat regular steel sheets with zinc. The zinc coating subsequently makes the steel sheets corrosion resistant. In other words, they are less susceptible to rust. If you expose regular steel to moisture, it rusts. The exposure may be in the form of ambient humidity or rain.
Rust or oxidation eventually corrodes regular steel to the point of failure. There are two anti-rust options. First, coat the steel to protect it from water, and second, use a metal that water does not corrode.
Which option a manufacturer chooses usually depends on cost.
The duration of the rust resistance depends on the thickness of the zinc layer. However, the thicker the layer, the more expensive it is.
WenzelMetalSpinning.com, a metal spinning company, based in Alabama, has the following definition:
“Galvanized steel is regular steel sheets that have been coated in zinc to make them corrosion resistant.”
”Regular steel is made of iron which will rust when exposed to moisture, either in the form of rain or ambient humidity.”
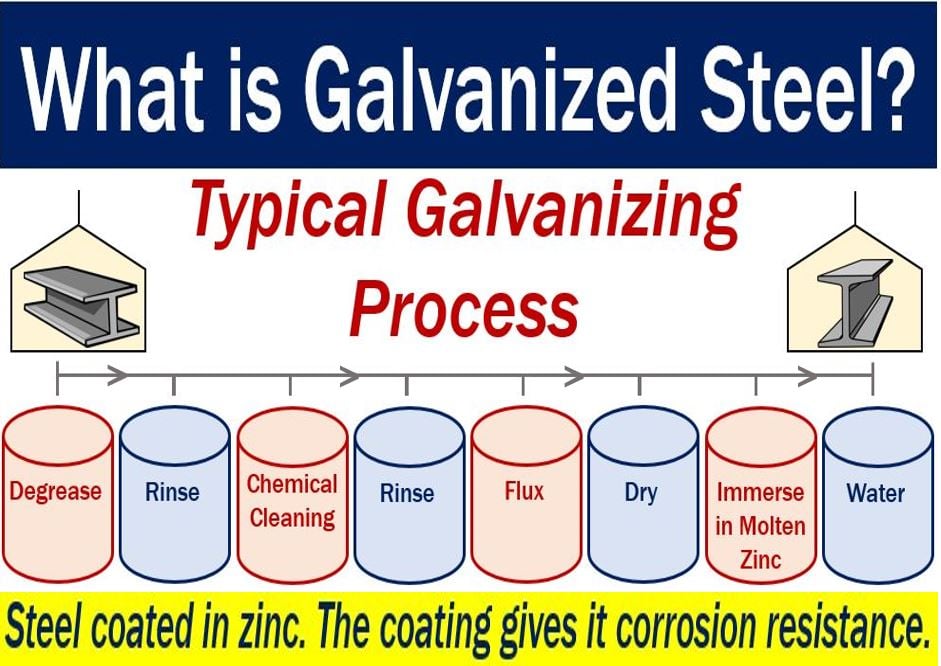
Galvanized steel – different processes
There are several processes for making galvanized steel. Below, you can read about three of them.
Hot-dip galvanizing
Hot-dip galvanizing deposits a thick layer of zinc-iron alloys on the steel’s surface.
The automotive industry uses a thinner form of galvanizing, i.e., electrogalvanizing. Vehicle bodies do not need a very thick layer of zinc because the manufacturer also adds coats of paint.
In most cases, hot-dip galvanizing does not weaken the steel on a measurable scale. However, hot-dip galvanizing of high-strength steels can result in hydrogen embrittlement. This type of weakening is something manufacturers of wire rope and other highly-stressed products must consider.
For steel products with constant exposure to corrosive materials, hot-dip galvanizing is insufficient. Acid, for example, is a corrosive material. In fact, even acid rain can cause hot-dip galvanized steel to rust.
For these applications, we need more expensive stainless steel.
Thermal diffusion galvanizing
Thermal diffusion galvanizing provides a zinc diffusion coating on copper- or iron-based metals. We also call this process Sherardizing. As the process involves no liquids, some people call it dry galvanizing.
Sherardizing involves tumbling parts and zinc powder in a sealed, rotating drum. At a temperature of 572 °F (300 °C), zinc diffuses into the substrate to form a zinc allow. The substrate is the underlying layer.
We typically find this type of galvanized steel in small, complex-shaped metals.
Thermal diffusion galvanizing is also useful for smoothing rough surfaces on products formed with sintered metal. Sintering is the process of forming a solid mass of material by heat, but without turning it into a liquid.
Electroplated galvanized steel
Electroplated galvanized steels have the pure zinc outer layer. However, they lack the intermediate alloy layer that mixes the zinc and steel.
Regarding this process, Zahner, an architectural metal and glass company, with headquarters in Kansas City, writes:
“Galvanized surfaces will lose the spangle and form a gray zinc oxide film with a slight tooth or fuzziness to them.
“Sometimes, streaks of red rust will develop on galvanized steel surfaces where the steel is no longer getting the benefit of the galvanic protection.”
Video – Hot-Dip Galvanizing
As this American Galvanizers Association explains, hot-dip galvanizing for corrosion protection has been around for decades.

