A team of researchers managed to make electric car batteries last longer by souping them up with fluorinated electrolytes. The scientists are from the University of Maryland (UMD), the US Army Research Laboratory (ARL), and Argonne National Laboratory (ANL). They say they have found a way to get more mileage out of electric cars.
To fluorinate means to introduce fluorine into a substance. Scientists can, for example, fluorinate a compound. Fluorine (F) is a chemical element – atomic number 9. Electrolytes are gels or liquids that contain ions that may be decomposed by electrolysis.
The success of an electric car battery depends on how far it can go on a single charge. In other words, one with a 400-mile range, for example, is superior to one with a 200-mile range.
Unfortunately, lithium-ion batteries are currently reaching their limit regarding how much charge one can pack into a given space.
Xiulin Fan, Long Chen, and colleagues wrote about their research in the prestigious journal Nature Nanotechnology (citation below). Co-first authors, Fan and Chen, are postdoctoral researchers at the A. James Clark School of Engineering, part of UMD.
#EV #batteries souped-up with fluorinated electrolytes for longer-range drivinghttps://t.co/7kDHSB9Rxg @ClarkSchool pic.twitter.com/OSZkQ9kSOi
— New Electronics (@New_Electronics) July 17, 2018
Fluorine-based electrolytes
Dr. Fan and Dr. Chen said:
“We have created a fluorine-based electrolyte to enable a lithium-metal anode, which is known to be notoriously unstable, and demonstrated a battery that lasts up to a thousand cycles with high capacity.”
The authors say that their new battery can charge and discharge several times over. They can do this without losing their ability to provide a reliable, constant, and high quality stream of energy.
In other words, you can charge and discharge their batteries again and again without virtually no loss of performance.
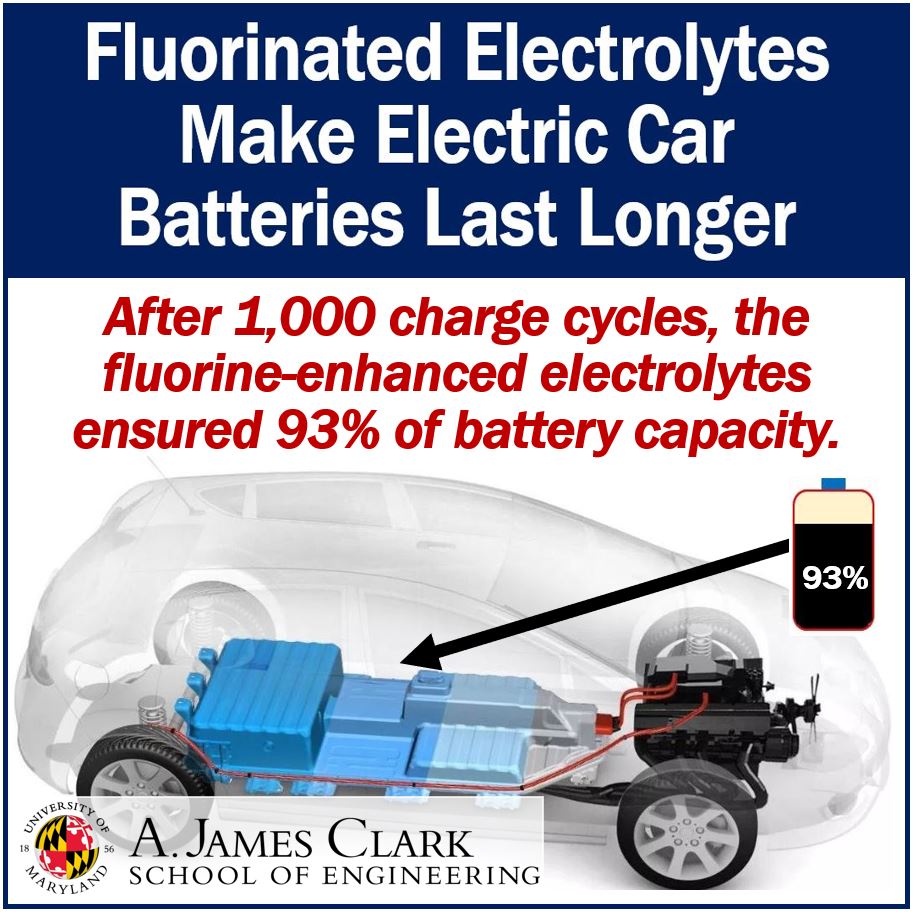
Fluorine enhanced electrolytes
Amazingly, even after 1,000 charge cycles, the battery has a capacity of 93%, thanks to the fluorine enhanced electrolytes. The authors described this capacity as ‘unprecedented.’
This means that a passenger car, for example, could run the same number of miles after each charge for several years.
Jang Wook Choi, an associate professor in chemical and biological engineering at Seoul National University, South Korea, said:
“The cycle lives they achieved with the given electrode materials and operation voltage windows sound ‘unprecedented.'”
“This work is a [sic] great progress forward in the battery field in the direction of increasing the energy density, although further tuning might be needed to meet various standards for commercialization.”
Prof. Choi was not part of the research team.
Using electrolytes for high-voltage batteries
The researchers demonstrated their batteries in coin-cell form, i.e., like watch batteries, for testing. They are currently working with partners in industry, specifically to use the electrolytes for high-voltage batteries.
We refer to lithium-metal anodes and high-voltage cathodes as ‘aggressive’ materials. Nickel is also ‘aggressive. We call them aggressive because they react strongly with other materials.
They can hold a lot of energy; however, they also devour the other elements they’re partnered with. Subsequently, those other elements become unusable.
Professor Chunsheng Wang has collaborated with Khalil Amine at ANL and Kang Xu at ARL. Specifically, they collaborated on the new electrolyte materials that improve battery performance. Prof. Wang works at the Department of Chemical and Biochemical Engineering at the Clark School.
The elements on the periodic table have different arrangements of electrons. Prof. Wang studies how each permutation of chemical structure might benefit or undermine a battery.
Prof. Wang and Prof. Xu are also the co-heads the Center for Research in Extreme Batteries. It is an industry-university-government collaborative effort. The effort aims to unite businesses that need batteries for unusual purposes with scientists who create them.
Electrolytes become non-flammable
Prof. Wang said:
“The aim of the research was to overcome the capacity limitation that lithium-ion batteries experience. We identified that fluorine is the key ingredient that ensures these aggressive chemistries behave reversibly to yield long battery life.”
“An additional merit of fluorine is that it makes the usually combustible electrolytes completely unable to catch on fire.”
The researchers filmed several batteries that caught fire. The fluorine battery, however, did not. In other words, it became non-flammable.
Prof. Xu said:
“You can find evidences from literature that either support or disapprove of fluorine as a good ingredient in interphases.”
“What we learned in this work is that, in most cases, it is not just what chemical ingredients you have in the interphase, but how they are arranged and distributed.”
“Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries,” Xiulin Fan, Long Chen, Oleg Borodin, Xiao Ji, Ji Chen, Singyuk Hou, Tao Deng, Jing Zheng, Chongyin Yang, Sz-Chian Liou, Khalil Amine, Kang Xu & Chunsheng Wang. Nature Nanotechnology – 16 July 2018. https://doi.org/10.1038/s41565-018-0183-2.
After a slow start, electric cars are becoming progressively more popular. A study last year predicted that by 2025, electric cars could displace 2m barrels of oil per day.
