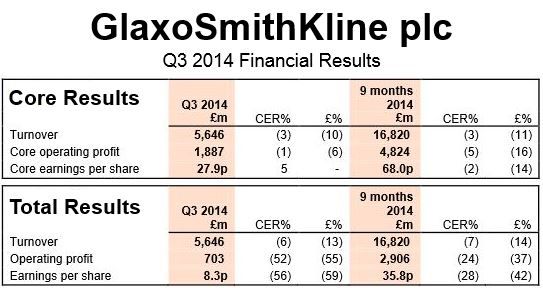
London-based pharmaceutical giant GlaxoSmithKline plc posted a pre-tax profit of £548 million for the third-quarter (ending September 30). Although this was a fraction of the £1.4 billion in Q3 2013, it was higher than analysts’ forecasts.
GlaxoSmithKline (GSK) pledged to give back to shareholders an additional £4 billion via a B share scheme after the proposed transaction with Novartis is completed.
GSK’s cost-cutting program is starting to pay off. With potentially lucrative new medications in the pipeline, the firm is upbeat regarding its outlook.
The company says it is aiming for a further £1 billion savings in annual costs over the next 36 months.
Over the past three months, GSK’s shares have declined by 14% compared to a 5% drop for the FTSE 100. Pricing pressure on Advair (asthma drug) in the US, plus a strong pound have contributed to the decline in shares.
The UK’s largest pharmaceutical company has had a year fraught with bribery and corruption accusations in China, Poland, Iraq, Jordan and some other countries. Earlier this year, the UK’s Serious Fraud Squad said it had launched a probe into GSK’s affairs. It has also had to deal with some major patent expiries.
(Data source: GlaxoSmithKline plc)
In order to “enhance visibility within the group,” the company is considering floating ViiV Healthcare, a firm that specializes in the development of HIV therapies that was created as a joint venture by GSK and Pfizer in 2009.
GSK reiterated its financial outlook for 2014, forecasting that core earnings will be about the same as last year.
Sir Andrew Witty, GSK’s CEO, said the company has been working towards completion of the proposed 3-part transaction with Novartis and expects it will be finalized in the first half of next year.
Regarding an Ebola vaccine, Sir Andrew said:
“With the current pressing public health emergency caused by spread of the Ebola virus in West Africa, I want to report to shareholders that we are working hard to develop a potential vaccine. This is still at an early stage, but we are grateful for the support of all our partners, including the World Health Organization, to expedite development of this candidate vaccine.”