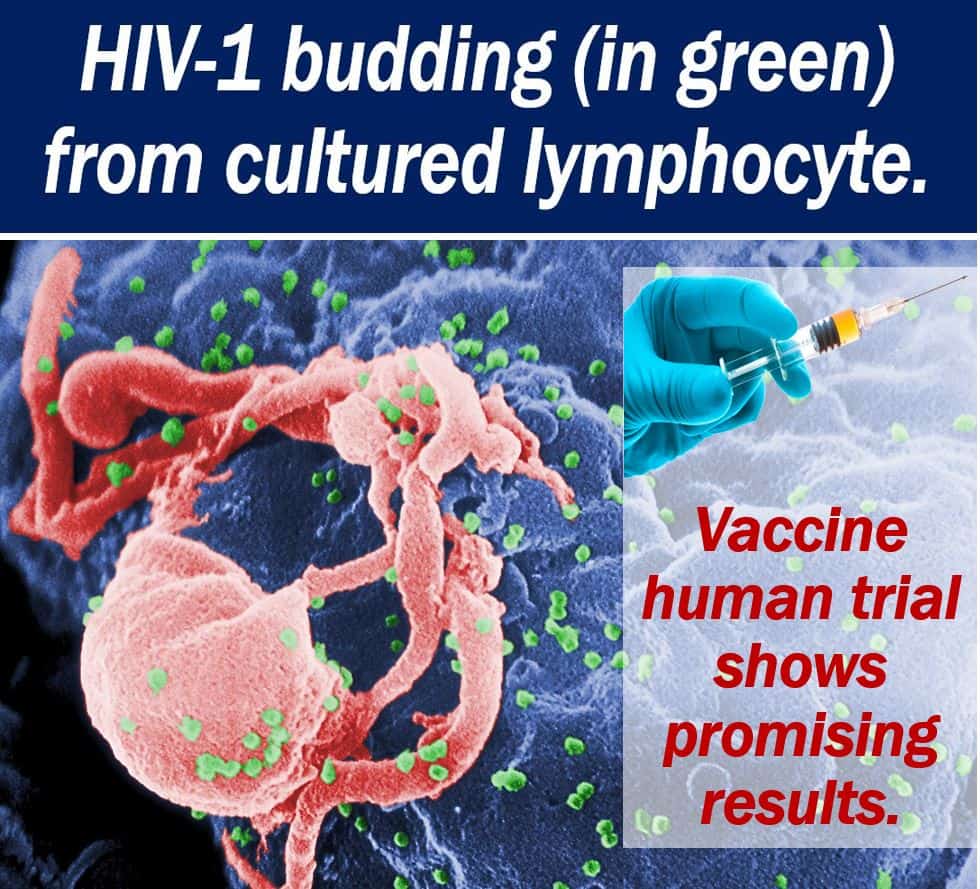
An HIV-1 vaccine human trial and a parallel trial with rhesus monkeys have shown promising results. The ‘mosaic’ HIV-1 vaccine has the potential to protect people globally from the virus. The human trial, which involved 393 participants, produced an anti-HIV immune system response.
The term ‘trial,’ in this context, refers to clinical trials, i.e., tests to determine how good and safe a drug or treatment is.
The researchers who carried out the trial published their findings in The Lancet.
Doctors diagnosed over 1.8 million new cases of HIV infection in 2016, the authors informed. We do not have a licensed prophylactic, i.e., intended to prevent disease, HIV vaccine.
So far, a major limitation has been the lack of direct comparability between preclinical studies and clinical trials. Clinical trials are medical experiments involving human beings.
In this study, the researchers aimed to evaluate mosaic adenovirus serotype 26-based HIV-1 vaccine candidates.
They carried out parallel studies in rhesus monkeys and humans to define “the optimal vaccine regimen to advance into clinical efficacy trials.”
There is a drug – Prep – which is effective at preventing HIV infection. However, people must take it regularly, i.e., sometimes daily, to protect themselves from the virus. A vaccine, on the other hand, involves just a single dose. Prep stands for pre-exposure prophylaxis.
HIV-1 vaccine – a huge challenge
Creating an effective HIV-1 vaccine has been a huge challenge for researchers. Partly because there are so many different HIV strains. The virus is also able to mutate, thus eluding attack from the human immune system.
However, this ‘mosaic’ HIV-1 vaccine has a multi-target approach. It consists of pieces of many different viruses. Therefore, it targets several different HIV strains.
Its creators believe it will offer significantly better protection. There are limitless HIV strains across the world.
Many HIV-1 vaccine combinations
In this randomized, double-blind, placebo-controlled human trial, researchers tested many different combinations of the mosaic vaccine.
The human participants, from Thailand, South Africa, Uganda, and Rwanda, ranged in age from 18 to 50 years.
Every vaccine combination produced an anti-HIV immune system response. All the combinations were also safe, the authors added.
Trials using humans and monkeys
The researchers conducted a parallel study with rhesus monkeys. They gave the monkeys the HIV-1 vaccine to protect against a simian-human immunodeficiency virus, i.e., HIV that infects monkeys.
Team leader, Dan H. Barouch, and colleagues reported that the vaccine protected 67% of the 72 monkeys from infection.
Dan H. Barouch, MD, PhD, is Director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center. He is also Professor of Medicine at Harvard Medical School.
Prof. Barouch said:
“This study demonstrates that the mosaic Ad26/Ad26 plus gp140 vaccine candidate induced robust and comparable immune responses in human and monkeys. Moreover, the vaccine provided 67 percent protection against viral challenge in monkeys.”
“Based on these data, the mosaic Ad26/Env HIV-1 vaccine has been advanced into a phase 2b clinical efficacy study to determine whether this vaccine will prevent HIV infection in humans in southern Africa.”
“We expect results in 2021. This is only the 5th HIV vaccine concept that will be tested for efficacy in humans in the 35+ year history of the global HIV epidemic.”
