The Nobel Prize in Chemistry 2016 went to three scientists who helped create the world’s smallest machines – a miniscule lift, artificial muscles and tiny motors. Jean-Pierre Sauvage, Sir J. Fraser Stoddart and Bernard L. Feringa are now Nobel laureates for their development of molecules with movements that can be controlled.
These tiny machines, nano-machines – the size of molecules – can perform tasks when energy is added.
These amazing molecular machines will probably be used in the development of new materials, energy storage systems, sensors, and hundreds of other exciting things.
 This year’s Nobel Prize in Chemistry 2016 winners – Prof. Jean-Pierre Sauvage (left), Sir J. Fraser Stoddart (centre), and Prof. Bernard L. Feringa (left) – for ‘taking chemistry to a new dimension’. (Image: twitter.com/NobelPrize)
This year’s Nobel Prize in Chemistry 2016 winners – Prof. Jean-Pierre Sauvage (left), Sir J. Fraser Stoddart (centre), and Prof. Bernard L. Feringa (left) – for ‘taking chemistry to a new dimension’. (Image: twitter.com/NobelPrize)
Sir J. Fraser Stoddart, a Scottish chemist, is a Board of Trustees Professor of Chemistry and head the Stoddart Mechanostereochemistry Group in the Department of Chemistry at Northwestern University, Evanston, Illinois, USA.
Bernard L. Feringa, a Dutch synthetic organic chemist, is the Jacobus van ‘t Hoff Distinguished Professor of Molecular Sciences at the Stratingh Institute for Chemistry, University of Groningen, The Netherlands. He is also an Academy Professor and Chair of Board of the Science Division of the Royal Netherlands Academy of Sciences.
Jean-Pierre Sauvage, a French coordination chemist who specializes in supramolecular chemistry, works as an emeritus professor of the University of Strasbourg, France, and is also a member of the Académie des Science.
Taken chemistry to a new dimension
The development of computing shows how the miniaturization of technology can eventually lead to a remarkable revolution. According to Nobelprize.org:
“The 2016 Nobel Laureates in Chemistry have miniaturised machines and taken chemistry to a new dimension.”
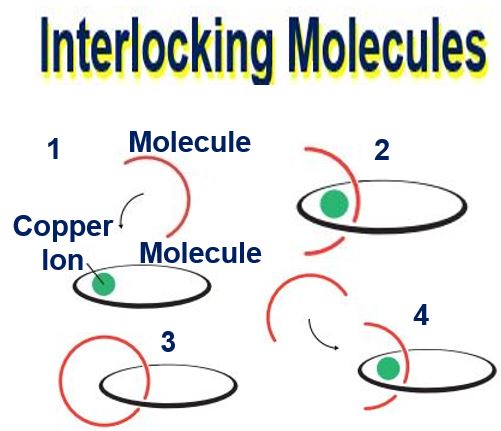 Prof. Sauvage used a copper ion to interlock molecules using a mechanical bond. 1. The molecules that will form a chain are attracted to a copper ion. 2. The copper ion gathers the molecules. 3. A 3rd molecule is linked to the crescent-shaped molecule. 4. The molecules are linked by a mechanical bond. The copper ion is removed. (Image: nobelprize.org)
Prof. Sauvage used a copper ion to interlock molecules using a mechanical bond. 1. The molecules that will form a chain are attracted to a copper ion. 2. The copper ion gathers the molecules. 3. A 3rd molecule is linked to the crescent-shaped molecule. 4. The molecules are linked by a mechanical bond. The copper ion is removed. (Image: nobelprize.org)
1. Catenane: Prof. Sauvage took the first step towards a molecular machine in 1983 when he managed to link two ring-shaped molecules together to form a catenane (a chain). Molecules are normally joined by strong covalent bonds in which the atoms share electrons. But in Sauvage’s chain they were linked by a freer mechanical bond.
For a machine to function properly and perform a task it must have parts that can move relative to each other. This requirement was fulfilled by the two interlocked rings.
2. Rotaxane: Prof. Stoddart took the second step in 1991, when he developed a rotaxane, a mechanically interlocked molecular architecture. He threaded a molecular ring onto a very thin molecular axle and showed that the ring could move along the axle.
Among Prof. Stoddart’s developments based on rotaxanes are a molecular muscle, a molecule-based computer chip, and a molecular lift.
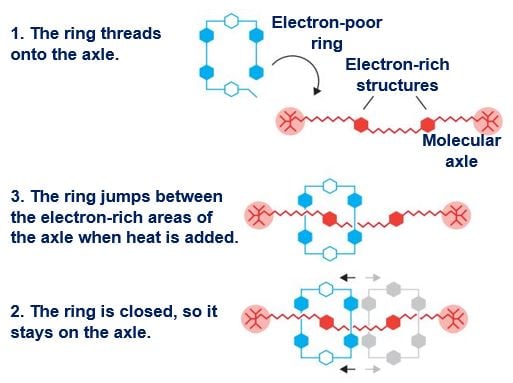 Prof. Stoddart created a molecular shuttle that could move, in a controlled manner, along an axle. (Image: nobelprize.org)
Prof. Stoddart created a molecular shuttle that could move, in a controlled manner, along an axle. (Image: nobelprize.org)
Prof. Stoddard said he first heard about his Nobel Prize at 4 a.m., when the telephone rang. Initially he thought it was a hoax, but soon realized it was for real when he recognized the Swedish-accented English.
Prof. Stoddard said:
“I am thrilled to be sharing this great honor with Jean-Pierre and Ben. We are as tight as thieves.”
3. Molecular Motor: In 1999, Prof. Feringa became the first individual to develop a molecular motor. He managed to get a molecular rotor blade to spin in the same direction continually. With the use of molecular motors, he rotated a glass cylinder that was ten-thousand times larger than the motor. He also designed a nanocar.
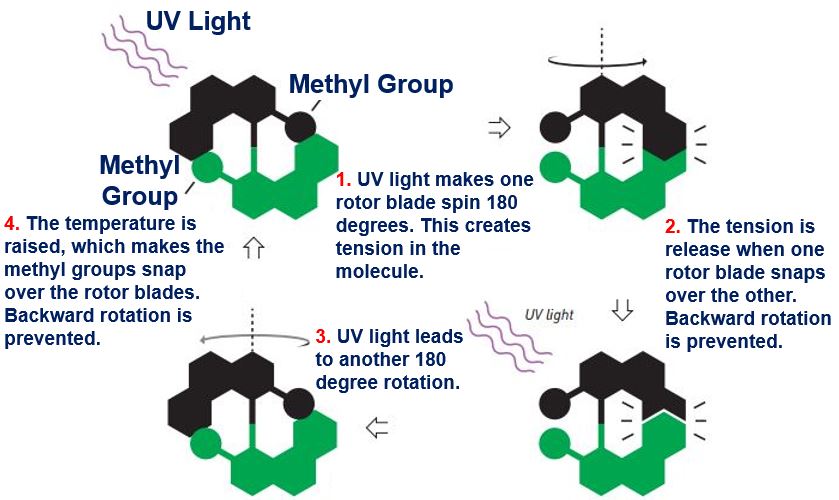 Prof. Feringa’s first molecular motor. (Image: nobelprize.org)
Prof. Feringa’s first molecular motor. (Image: nobelprize.org)
Prof. Feringa first heard the news while in his office at the university. It soon filled with colleagues and students who congratulated him. Prof. Feringa said:
“I am deeply honoured. This is all thanks to the hard work of all you smart students and staff here. Thank you. And I hope this will help you in your future careers too.”
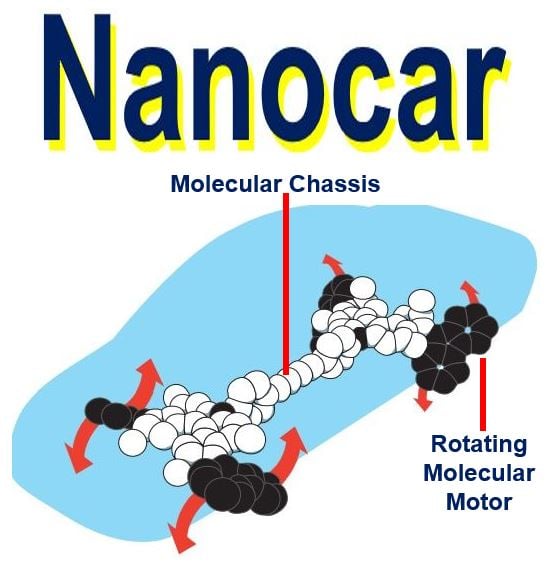 Prof. Feringa’s four-wheel drive nanocar. He and his team built it in 2011. A molecular chassis held together four motors that served as wheels. When the wheels rotated, the car moved forward over a surface. (Image: nobelprize.org)
Prof. Feringa’s four-wheel drive nanocar. He and his team built it in 2011. A molecular chassis held together four motors that served as wheels. When the wheels rotated, the car moved forward over a surface. (Image: nobelprize.org)
Nobelprize.org added:
“2016’s Nobel Laureates in Chemistry have taken molecular systems out of equilibrium’s stalemate and into energy-filled states in which their movements can be controlled.”
“In terms of development, the molecular motor is at the same stage as the electric motor was in the 1830s, when scientists displayed various spinning cranks and wheels, unaware that they would lead to washing machines, fans and food processors. Molecular machines will most likely be used in the development of things such as new materials, sensors and energy storage systems.”
Michel Deneken, interim president of the University of Strasbourg, where Prof. Sauvage works, said:
“We proudly received theis great news. The honour we share with the CNRS will have a positive effect on our whole site. This distinction rewards the pioneer work on the design and synthesis of molecular machines. These nanoscale compilations are capable of actuating movement in a controlled manner as response to diverse signals as UV light.”
Video – Nobel Prize in Chemistry 2016
In this Nobel Prize video, you can see the announcement of the three new Nobel Chemistry Laureates at the Royal Swedish Academy of Sciences, and a brief explanation of their research.

Comments are closed.