Burning carbon-based fuels to produce energy gives off carbon dioxide, a greenhouse gas that contributes to global warming. Now, scientists at the US Department of Energy’s Oak Ridge National Laboratory have discovered – almost by accident – an efficient way to convert CO2 into ethanol, a carbon-based fuel.
“We’re taking carbon dioxide, a waste product of combustion, and we’re pushing that combustion reaction backwards with very high selectivity to a useful fuel,” says one of the researchers, Dr. Adam Rondinone.
He and his colleagues at Oak Ridge were experimenting with nanotechnology to make new catalysts out of inexpensive materials to do the work of more expensive catalysts.
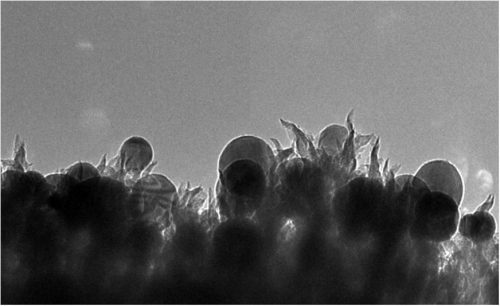 The researchers created a new type of catalyst comprising copper nanoparticles (the spheres) embedded in carbon nanospikes that converts CO2 into ethanol. Image: Oak Ridge National Laboratory
The researchers created a new type of catalyst comprising copper nanoparticles (the spheres) embedded in carbon nanospikes that converts CO2 into ethanol. Image: Oak Ridge National Laboratory
Catalysts are substances that enable chemical reactions without being present in the products of the reactions – they just have to be there. They are usually made from expensive and rare materials, such as platinum.
Nanotechnology involves working with matter at the atomic and molecular scale to create materials with remarkable and unusual properties. These nanoscale properties are often quite different to how the materials behave at the normal scale.
Unusual nanoscale structure
The Oak Ridge team was working with a catalyst made of carbon, copper, and nitrogen fabricated into an unusual nanoscale structure and found it could convert CO2 dissolved in water into ethanol with a high yield of 63 percent.
Usually, this type of electrochemical reaction gives a mix of products in small amounts. The team believes it is the unusual properties of the nanoscale structure that produces the high yield of ethanol.
The nanoscale structure comprises copper nanoparticles embedded in carbon nanospikes. When they analyzed what was happening, the researchers found the spiky textured surface of the catalyst offered plenty of sites for the conversion of CO2 into ethanol.
“They are like 50-nanometer lightning rods that concentrate electrochemical reactivity at the tip of the spike,” says Rondinone, an expert on materials chemistry at the nanoscale.
Store electricity
Because the process they have developed uses low-cost materials, and operates at room temperature in water, Rondinone and colleagues believe it could be scaled up for industrial use.
They suggest one application could be to store any extra electricity that is generated from variable power sources such as solar and wind farms.
“This could help to balance a grid supplied by intermittent renewable sources,” Rondinone explains.
The team reports the work in a paper published in the journal Chemistry Select.
Video – converting CO2 into ethanol
In the following video from Oak Ridge National Laboratory, Rondinone and colleagues describe the electrochemical process they developed that uses a nanostructure of carbon and copper to turn CO2 into ethanol.

