Scientists have developed an e-waste recycling process that can effectively separate polymers from the mixed plastics of discarded electronic products – such as cellphone cases – so that they can be re-used.
Many parts of electronic waste, or e-waste, from products such as cellphones, video game consoles, refrigerators, computers, and televisions, are not difficult to recycle – for example metal and glass.
 When it comes to e-waste recycling, mixed-polymer plastics – such as cellphone cases – often end up in landfill as they are some of the least easy materials to recycle. Image: CP/pixabay composite.
When it comes to e-waste recycling, mixed-polymer plastics – such as cellphone cases – often end up in landfill as they are some of the least easy materials to recycle. Image: CP/pixabay composite.
And plastic products such as water bottles and milk containers are also readily recyclable because they are made of single polymers.
But e-waste recycling of plastics has proved to be more challenging because electronic products contain more complex, mixed polymers.
Now, a study from the University of Illinois Urbana-Champaign and published in the journal ACS Sustainable Chemistry & Engineering, describes for the first time, an e-waste recycling process that converts mixed plastics into reusable polymers in an environmentally-friendly and energy-efficient way.
Senior study author Brajendra Kumar Sharma, who is a senior research scientist at the University’s Illinois Sustainable Technology Center (ISTC), says that because they contain more complex blends of polymers, plastic items like cellphone cases usually “end up being incinerated or sent to landfills because there are no safe or efficient means of recycling them.”
‘Like making candy’
The process for recovering polymers by dissolving them in a solvent is not unlike making candy out of sugar, explains fellow researcher and study lead author Sriraam R. Chandrasekaran, who is a lead research engineer at ISTC.
“With candy,” he continues, “you dissolve sugar grains in water, allow the liquid to become saturated with sugar, evaporate the water and recover the polymer, which in this case is the candy.”
The difference, he adds, is that in their lab they use plastic polymers in the place of sugar and the solvent that they use is stronger than water.
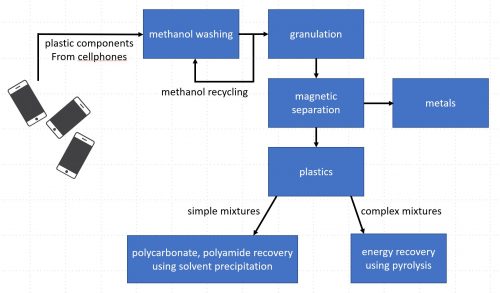
The study describes two processes: solvent extraction of simple mixtures such as polycarbonate and polyamide from cellphone plastics, and energy recovery from pyrolysis of complex mixtures from other e-waste materials. Image: adapted from Sriraam R. Chandrasekaran et al, 2018
The most efficient industrial process uses a solvent called dichloromethane (DCM). However, this releases substances that can cause cancer into the air, even at temperatures not far off those of room temperature.
Chandrasekaran says that their process uses a solvent called N-methyl-2-pyrrolidone (NMP), “which will only release vapors when heated to 180 degrees Celsius, far above the temperature needed to dissolve the polymers.”
Near virgin-material quality
The process starts with methanol washing of the e-waste plastic, such as cellphone cases. The methanol in this stage is reused again and again.
After methanol washing, the plastic is broken up into granules and any metal bits are recovered.
For simple mixtures – such as polycarbonate (PC)/polyamide (PA) mixtures found in cellphone plastics – the process used is a solvent extraction to recover the pure polycarbonate and polyamide.
The study showed that it is possible, in the laboratory, to make small quantities of the recovered polymers with a quality that is comparable to their original virgin materials.
Re-using process materials
An important feature of the e-waste recycling process is that – like the methanol recycling – it can re-use the evaporated NMP again and again by condensing it.
Sharma says that e-waste recycling studies like theirs aim not only to recycle the target waste, but also to do so in a manner that is efficient and environmentally friendly.
More complex mixtures – the authors give the example of “PC/PA/acrylonitrile butadiene styrene (ABS)/poly methyl methacrylate (PMMA)” – undergo pyrolysis and are converted into an oil that can be used as fuel.
“Ideally,” says Chandrasekaran, “we would like to chop that step off and have the solvent process end in a complete recycle.”
The team now wants their recycled polymers to be used as the starting material for a manufacturing process so that they can see whether they are of sufficiently high quality.
“If successful, we can then begin a pilot-scale project,” Sharma concludes.

