Synthetic carbyne, which is assumed to be twice as stiff as graphene and forty times stiffer than diamond, can be produced synthetically, says a group of scientists from Austria, Germany, Japan, Turkey and Spain. They have found a way to mass produce carbon chains consisting of 6,400 carbon atoms.
The scientists explained in the prestigious journal Nature Materials (citation below) that before this major breakthrough in the world of carbon research, the record number of carbon atoms synthesized in a single chain was just one hundred.
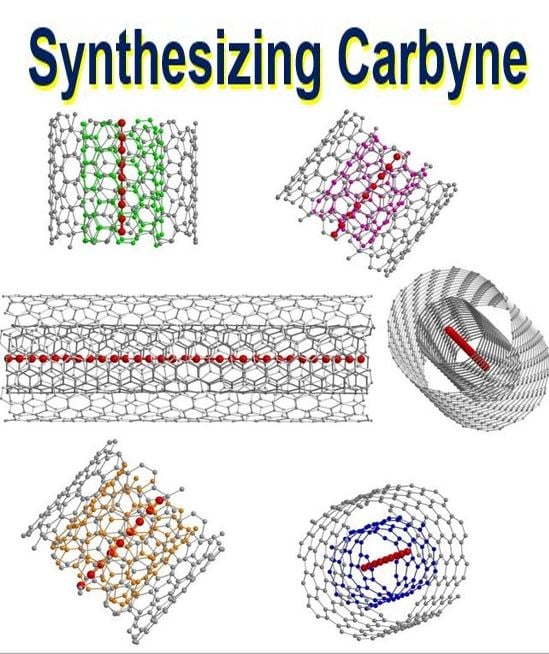 An artist’s schematic representation of confined ultra-long linear acetylenic carbon chains inside different double-walled carbon nanotubes. (Image: Max Planck Institute. Credit: Lei Shi, University of Vienna)
An artist’s schematic representation of confined ultra-long linear acetylenic carbon chains inside different double-walled carbon nanotubes. (Image: Max Planck Institute. Credit: Lei Shi, University of Vienna)
Carbyne has long been the elusive one
Elemental carbon appears in several forms, including diamond, fullerenes and graphite. Their unique structural, optical, mechanical and transport properties have a wide range of applications in chemistry, physics, nanoelectronics and materials science, including energy-harvesting materials and light-emitting devices.
Only carbyne – the truly 1-dimensional form of carbon – within this ‘carbon family’ has not yet been synthesized (made artificially). Despite several attempts by scientists across the world for the past five decades, its extreme instability in room temperature has made it impossible for researchers to provide the final experimental proof of its existence.
Head of Electronic Properties of Materials at the University of Vienna, Prof. Thomas Pichler, who led the study, and colleagues have succeeded in developing a new route for the mass-production of carbon chains composed of over 6,000 carbon atoms.
They used thin double-walled carbon nanotubes as the chains’ protective hosts.
 Nanorods (nanoropes) of carbyne. Researchers have long been trying to make the carbon-atom chains. (Image: news.rice.edu)
Nanorods (nanoropes) of carbyne. Researchers have long been trying to make the carbon-atom chains. (Image: news.rice.edu)
Closer to mass-producing carbyne
The authors say their findings represent an elegant prelude before the final goal of mass-producing carbyne – a feat that will be published in Nature Materials.
Even in its elemental form (at its basic or normal state without combination of other items), the high bond versatility of carbon allows for several different well-known materials, including graphite and diamond.
Graphene – a single layer of graphite – can then be folded or rolled into fullerenes or nanotubes respectively. So far, Nobel prizes have been awarded for both fullerenes (1966) and graphene (2010).
Although carbyne’s existence – an infinitely long carbon chain – had been proposed in 1885 by Adolf von Baeyer (1835-1917), a German chemist who was awarded the Nobel Prize in 1905, scientists have not yet succeeded in synthesizing this material.
Von Baeyer said that as carbyne’s high reactivity would always lead to its immediate destruction, it would be a perpetually-elusive material.
Over the past fifty years, however, carbon chains of increasing length have been synthesized successfully – in 2003, a record of about 100 carbon atoms was achieved.
In this new paper, the authors claim they have broken that record by more than one order of magnitude, with the demonstration of micrometre length-scale chains.
A new record
Prof. Pichler and his team have presented a new approach to grow and stabilize carbon chains with a record length of 6,400 carbon atoms. They used the confined space within a double-walled carbon nanotube as a nano-reactor to grow incredibly-long carbon chains on a bulk scale.
Together with scientists from the AIST Nanotube Research Centre in Japan, the Universidad del País Vasco in Spain, the UNAM-National Nanotechnology Research Center in Turkey, the Max Planck Institute for the Structure and Dynamics of Matter in Germany, and ETH Zürich’s Photonics Laboratory in Switzerland, the existence of chains has been compellingly and unambiguously confirmed by using an array of sophisticated, complementary methods.
The complementary methods included temperature-dependent near- and far-field Raman spectroscopy with different lasers, for research into electronic and vibrational properties; x-ray scattering, for the confirmation of bulk chain growth; and high-resolution transmission electron spectroscope, to directly observe carbyne inside the nanotubes.
The Holy Grail of carbon allotropes
Lead author, Lei Shi, who works at the University of Vienna, explained:
“The direct experimental proof of confined ultra-long linear carbon chains, which are more than an order of magnitude longer than the longest proven chains so far, can be seen as a promising step towards the final goal of unraveling the “holy grail” of carbon allotropes, carbyne.”
When inside the double-walled carbon nanotubes, carbyne is very stable. This property is vital for its eventual application in future devices and materials.
Theoretical models suggest that carbyne’s mechanical properties exceed every single material we know about. It outperforms both diamond and graphene. The authors believe carbyne’s electrical properties have potential for new nanoelectronic applications in magnetic semiconductors and quantum spin transport.
In an Abstract in the journal, the authors wrote:
“The synthesis of very long arrangements is confirmed by a combination of transmission electron microscopy, X-ray diffraction and (near-field) resonance Raman spectroscopy.”
“Our results establish a route for the bulk production of exceptionally long and stable chains composed of more than 6,000 carbon atoms, representing an elegant forerunner towards the final goal of carbyne’s bulk production.”
Citation: “Confined linear carbon chains as a route to bulk carbyne,” Lei Shi, Philip Rohringer, Kazu Suenaga, Yoshiko Niimi,Jani Kotakoski, Jannik C. Meyer, Herwig Peterlik, Marius Wanko, Seymur Cahangirov, Angel Rubio, Zachary J. Lapin, Lukas Novotny, Paola Ayala, Thomas Pichler, Nature Materials. 4 March 2016. DOI: 10.1038/nmat4617.
