A major artificial photosynthesis breakthrough could be a huge game-changer for addressing human-induced climate change by capturing CO2 emissions before they are vented into the atmosphere.
Scientists at the Lawrence Berkeley National Laboratory (Berkeley Lab), part of the Department of Energy, and the University of California Berkeley explained that the CO2 is converted into valuable chemical products, including liquid fuels, pharmaceutical medications and biodegradable plastics – using solar energy.
The researchers, who wrote about their breakthrough in the academic journal Nano Letters (citation below), say they have created a hybrid system of bacteria and nanowires that mimics the way plants use the energy in sunlight to synthesize carbohydrates from CO2 and water, i.e. photosynthesis.
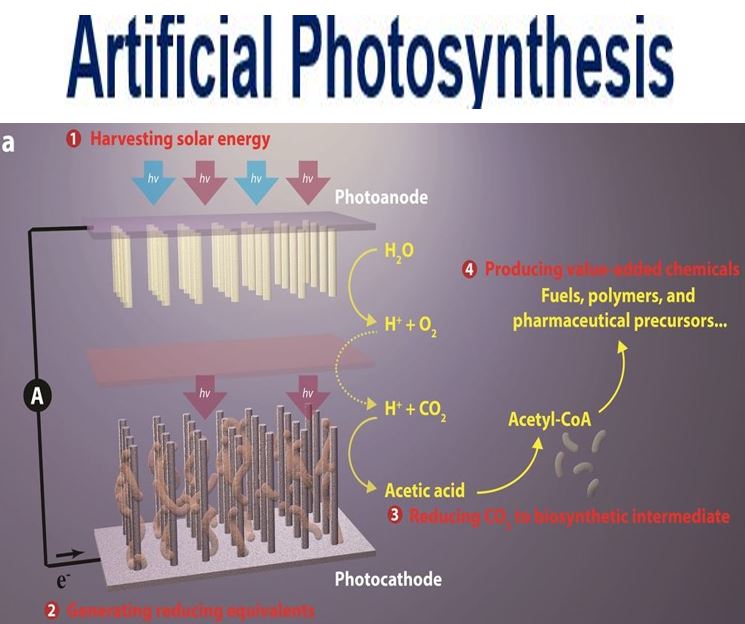
This break-through artificial photosynthesis system has four general components: 1. Harvesting solar energy. 2. Generating reducing equivalents. 3. Reducing CO2 to biosynthetic intermediates. 4. Producing value-added chemicals. (Image: Berkeley Lab)
However, in this case, the artificial photosynthetic system synthesizes the combination of H2O and CO2 into acetate, which is currently the most common building block for biosynthesis (the production of complex molecules within living organisms or cells).
Co-author and one of the study leaders, Peidong Yang, a chemist with Berkeley Lab’s Materials Sciences Division, said:
“We believe our system is a revolutionary leap forward in the field of artificial photosynthesis. Our system has the potential to fundamentally change the chemical and oil industry in that we can produce chemicals and fuels in a totally renewable way, rather than extracting them from deep below the ground.”
Earth’s atmosphere’s temperature is closely associated with how much CO2 is released – more CO2 means warmer temperatures.
Atmospheric CO2 is today at its highest level in at least 3 million years, mainly due to the burning of fossil fuels.
Cannot shut down fossil fuels, we need it
However, fossil fuels, particularly coal, will continue being a major source of energy to meet human requirements for the foreseeable future.
The problem with current technologies being studied or suggested for sequestering carbon, is that they all require that captured carbon be stored, which creates another environmental problem of its own.
With the artificial photosynthesis system created by the Berkeley scientists, the storage problem is solved by putting the captured CO2 into good use.
Chris Chang, an expert in catalysts for carbon-neutral energy conversions, said:
“In natural photosynthesis, leaves harvest solar energy and carbon dioxide is reduced and combined with water for the synthesis of molecular products that form biomass.”
“In our system, nanowires harvest solar energy and deliver electrons to bacteria, where carbon dioxide is reduced and combined with water for the synthesis of a variety of targeted, value-added chemical products.”
A win-win situation for the environment
By combining select bacterial populations with biocompatible light-capturing nanowire arrays, the new system that mimics what plants do offers a win-win situation for the environment, i.e. solar-powered green chemistry utilizing sequestered CO2.
Michelle Chang, an expert in biosynthesis, said:
“Our system represents an emerging alliance between the fields of materials sciences and biology, where opportunities to make new functional devices can mix and match components of each discipline.”
“For example, the morphology of the nanowire array protects the bacteria like Easter eggs buried in tall grass so that these usually-oxygen sensitive organisms can survive in environmental carbon-dioxide sources such as flue gases.”
The system begins with an artificial forest of nanowire heterostructures, made of titanium oxide and silicon nanowires, which Yang and his research group had previously developed.
Yang said:
“Our artificial forest is similar to the chloroplasts in green plants. When sunlight is absorbed, photo-excited electron-hole pairs are generated in the silicon and titanium oxide nanowires, which absorb different regions of the solar spectrum.”
“The photo-generated electrons in the silicon will be passed onto bacteria for the CO2 reduction while the photo-generated holes in the titanium oxide split water molecules to make oxygen.”
Nanowire arrays plus bacteria combinded lower CO2
When the forest of nanowire arrays has been established, it is populated with select populations of bacteria – Sporomusa ovata – that produce enzymes known to selectively catalyze the reduction of CO2.
Sporomusa ovata is an anaerobic bacterium that readily accepts electrons directly from the environment around it and uses them to reduce CO2.
Michelle Chang said:
“S. ovata is a great carbon dioxide catalyst as it makes acetate, a versatile chemical intermediate that can be used to manufacture a diverse array of useful chemicals.”
“We were able to uniformly populate our nanowire array with S. ovata using buffered brackish water with trace vitamins as the only organic component.”
Genetically-engineered bacteria make chemical products
After the CO2 has been reduced by the bacterium to acetate, or some other biosynthetic intermediate, genetically engineered E. coli are used to synthesize targeted chemical products.
In order to get the best yields of targeted chemical products, the E. coli and S. ovata were kept apart for this study.
These two activities – catalyzing and synthesizing – could in future be combined into a single-step process.
A key to the success of this artificial photosynthesis system is the separation of the challenging requirements for light-capture efficiency and catalytic activity, which is made possible by the bacteria/nanowire hybrid technology.
With this technology, the Berkeley scientists achieved a solar energy conversion efficiency of up to 0.38% for about 200 hours under simulated sunlight, i.e. about the same efficiency as that of a leaf.
The scientists were also encouraged by the yields of target chemical molecules produced from the acetate – up to 26% for butanol, a fuel similar to gasoline, and 25% for amorphadiene, a precursor for anti-malarial medication artemisinin, and 52% for the renewable, and biodegradable plastic PHB.
As the technology is refined further, the authors expect performances to improve.
Yang said:
“We are currently working on our second generation system which has a solar-to-chemical conversion efficiency of three-percent.”
“Once we can reach a conversion efficiency of 10-percent in a cost effective manner, the technology should be commercially viable.”
Most of the funding for this research came from the DOE Office for Science.
Citation: “Nanowire–Bacteria Hybrids for Unassisted Solar Carbon Dioxide Fixation to Value-Added Chemicals,” Chong Liu, Joseph J. Gallagher, Kelsey K. Sakimoto, Eva M. Nichols, Christopher J. Chang, Michelle C. Y. Chang and Peidong Yang. Nano Letters. Published 17 April, 2015. DOI: 10.1021/acs.nanolett.5b01254.

