Scientists at Monash University in Australia say that proteins usually linked to the destruction of cancerous or virally infected cells in the human immune system have been found in fruit flies (Drosophila melanogaster). The proteins control the fruit fly’s cell release of a critical growth factor that governs the embryo’s head and tail development.
The researchers, who published their findings in the academic journal Nature Communications, believe this may help explain how these perforin-like proteins function in the development of the human brain and neurodevelopment disorders such as Autism Spectrum Disorder.
The study was carried out by Dr. Michelle Henstridge, a post-doctoral researcher, Dr. Travis Johnson, an Australian Research Council DECRA Fellow, and co-led by Coral Warr, Associate Professor at the School of Biological Sciences, and James Whisstock, Professor in the Department of Biochemistry and Molecular Biology.
 Prof. Coral Warr (left) and the research team. (Image: Monash University)
Prof. Coral Warr (left) and the research team. (Image: Monash University)
Their finding solves a long-standing question in developmental biology: how a fly embryo’s growth factor is controlled to determine where the tail and head form.
New mechanism for controlling growth factor activity
Dr. Johnson said:
“These findings are significant and exciting as they suggest a completely new mechanism for how growth factor activity can be controlled.”
Dr Henstridge added:
“Understanding how growth factor activity is controlled is vital because loss of control of growth factors underlies many of the major diseases that afflict society, such as cancer and obesity.”
The fruit fly’s perforin-like protein is called ‘Torso like’ because female flies that don’t have this protein produce embryos with no heads or tails.
Prof. Warr explained why the fruit fly is the perfect model for studying the role perforin-like proteins play in development.
Prof. Warr said:
“The fruit fly Drosophila is a fantastic organism for investigating the question of how these perforin-like proteins act in embryo development; most of our knowledge of how human development and growth is regulated started with studies in the fruit fly. This is because most human genes controlling development and growth act in the same way in fruit flies.”
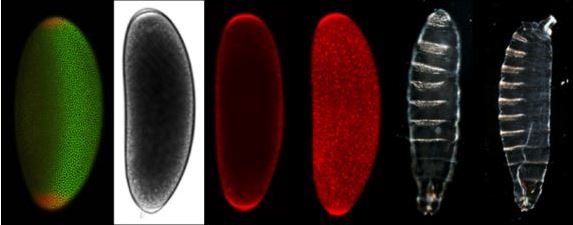 From left to right: 1. Fruit fly embryo stained for markers of localised patterning. 2. Brightfield microscopy view of a young embryo. 3. Confocal section of fruit fly embryo expressing the growth factor for required patterning of the head and tail. 4. Whole fruit fly embryo confocal projections image. 5. Fruit fly larva mutant for the torso-like gene with head and tail defects. 6. Normally patterned larva. (Image: Centre for Advanced Molecular Imaging)
From left to right: 1. Fruit fly embryo stained for markers of localised patterning. 2. Brightfield microscopy view of a young embryo. 3. Confocal section of fruit fly embryo expressing the growth factor for required patterning of the head and tail. 4. Whole fruit fly embryo confocal projections image. 5. Fruit fly larva mutant for the torso-like gene with head and tail defects. 6. Normally patterned larva. (Image: Centre for Advanced Molecular Imaging)
Prof. Whisstock went on to explain the significance for us of discovering how a protein related to perforin, that helps the human immune system kill foreign pathogen cells, is used to release a growth factor only at each end of an insect’s embryo.
Prof. Whisstock said:
“What’s exciting about our research is the discovery that a protein related to perforin – which usually functions to kill cells – is actually helping cells develop and differentiate in fly embryos. This is important because a group of perforin-like proteins found in the human brain have, in previous research, been shown to be associated with proper brain development.”
Prof. Warr added:
“While we don’t yet know how these proteins work, we suspect they may also be involved in controlling growth factor release from cells.”
The scientists believe their research may lead to opportunities for the creation of new treatments, for example, to treat Autism Spectrum Disorder and other developmental disorders and conditions.
They also aim to continue studying fruit flies to explore the functions of important mammalian proteins on a much larger scale.
Citation: “Torso-like mediates extracellular accumulation of Furin-cleaved Trunk to pattern the Drosophila embryo termini,” Travis K. Johnson, Michelle A. Henstridge, Anabel Herr, Karyn A. Moore, James C. Whisstock & Coral G. Warr. Nature Communications. Published 28 October, 2015. DOI: 10.1038/ncomms9759.


Comments are closed.