Today, Google Doodle celebrates the 182nd birthday of Dmitri Mendeleev, known as the father of the Periodic Table – the scientist who made sense of the fifty-six elements known to science at the time. There is a painting of the famous scientist on Google’s home page today.
Dmitri Ivanovich Mendeleev (1834-1907) was a Russian chemist and inventor who formulated the Periodic Law, created a visionary version of the periodic table of elements, and used it to correct the properties of some elements that had already been discovered.
Mendeleev also predicted the properties of eight elements that had not yet been discovered.
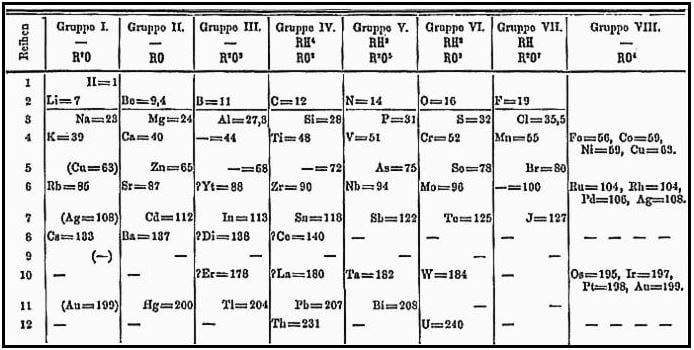 Dmitri Mendeleev created this Periodic Table in 1871. (Image: Wikipedia)
Dmitri Mendeleev created this Periodic Table in 1871. (Image: Wikipedia)
Elements organised differently throughout history
About 400 BC, the ancient Greeks organized the elements into four groups – fire, earth, water and air. In the seventeenth century, Robert Boyle (1627-1691), an Anglo-Irish natural philosopher, chemist, physicist and inventor, explained the material world in terms of elements, compounds and mixtures.
It was not until 1869, that Dimitri Mendeleev showed how the fifty-six elements that scientists knew of at the time were related to each other in a distinct pattern.
Mendeleev’s periodic table let elements fall into Periods, according to their atomic mass and valence (relating to or denoting electrons involved in or available for chemical bond formation, i.e. the power that determines how the elements combine).
Mendeleev’s grid more than a simple chart
Before Mendeleev, scholars had tried to organise the tables into elements, but what Mendeleev did extended way beyond mere chart-making.
Medneleev used logic in the table he created, which predicted the existence of other elements that had not yet been discovered, such as germanium and gallium. With his table, he was even able to predict their behaviours.
Not all the predictions were correct, however, but the fundamental principles behind his periodic organisation continue to stand at the foundation of chemistry today.
 Dmitri Ivanovich Mendeleev (Дми́трий Ива́нович Менделе́ев), known as the father of the Periodic Table, was born on February 8, 1834 in Tobolsk Governorate, Russian Empire. (Image: dmitrimendeleev.com)
Dmitri Ivanovich Mendeleev (Дми́трий Ива́нович Менделе́ев), known as the father of the Periodic Table, was born on February 8, 1834 in Tobolsk Governorate, Russian Empire. (Image: dmitrimendeleev.com)
Every schoolchild across the world today is drawn by the poster on the classroom wall of the periodic table in his or her first chemistry lesson. The periodic table today has 118 elements. The last one to be declared a new element was ununpentium in December 2015, after it was discovered by a group of nuclear scientists in Russia – it has a half-life of just 220 milliseconds.
Regarding Dmitri Mendeleev’s 182nd birthday, the Google Doodle blog wrote:
“In the final illustration, artist Robinson Wood imagines Mendeleev in the act of setting down the logic of his table (which reportedly came to him in a dream). Today, on Mendeleev’s 182nd birthday, we celebrate how this visionary helped us order and understand our world.”
Periodic Table inspired from a dream
After he became a teacher, Mendeleev wrote the book – Principles of Chemistry (2 volumes, 1868-1870). As he tried to classify all the elements according to their chemical properties, he noticed there were patterns which led him to create his Periodic Table.
Mendeleev said he envisioned the complete arrangement of all the elements in a dream.
Mendeleev said:
“I saw in a dream a table where all elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper, only in one place did a correction later seem necessary.”
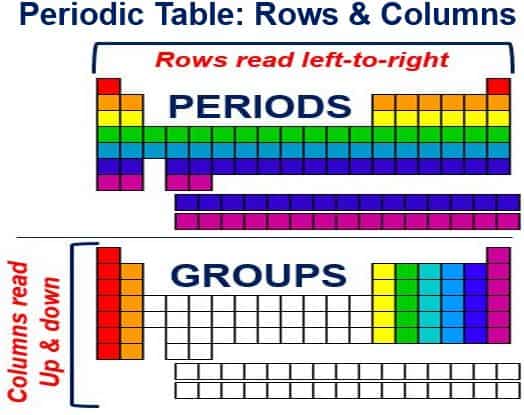 The Periodic Table is arranged into columns (periods) and rows (groups)
The Periodic Table is arranged into columns (periods) and rows (groups)
What is the Periodic Table?
The Periodic Table is like a large grid. Each element in it is placed in a specific location because of the structure of its atoms. The grid has columns (up and down) and rows (left to right). Each column and row has specific characteristics.
For example, Mg (magnesium) and Be) beryllium are in column two and share certain similarities, while Ca (calcium) and K (potassium), which are in row four, share other characteristics.
A Row is a Period: although they skip some squares in between, all the rows read from left to right. Each row on the Periodic Table is called a Period. All the elements in a row (period) have the same number of atomic orbitals. For example, in the first period (top row), every element has one orbital for its electrons.
 Today (8 Feb, 2016) Google Doodle celebrates Dmitri Mendeleev’s 182nd birthday with this image. (Image: google.co.uk/logos/doodles)
Today (8 Feb, 2016) Google Doodle celebrates Dmitri Mendeleev’s 182nd birthday with this image. (Image: google.co.uk/logos/doodles)
In the second period (second row), the elements have two orbitals for their electron – every row as you move down adds an orbital. There are currently no more than seven electron orbitals.
A Column is a Group: there are also vertical columns in the periodic table. Each column is known as a Group. The elements in each group (column) have the same number of electrons in the outer orbital. These outer electrons are also called valence electrons. These electrons are involved in chemical bonds with other elements.
In Group 1 (first column), each element has one electron in its outer shell, two electrons in Group 2, three in Group 3, etc. There are some exceptions, such as with the transition elements, which add electrons to the second-to-last orbital.
 The Mendeleev Medal: every two years, the Russian Chemical Society together with the Russian Academy of Sciences award the golden Mendeleev Medal for outstanding work in chemistry. (Image: Wikipedia)
The Mendeleev Medal: every two years, the Russian Chemical Society together with the Russian Academy of Sciences award the golden Mendeleev Medal for outstanding work in chemistry. (Image: Wikipedia)
Mendeleev’s other contributions to science
Mendeleev also made several other important contributions to chemistry. Lev Chugaev, the Russian chemist and science historian, characterized Mendeleev as:
“A chemist of genius, first-class physicist, a fruitful researcher in the fields of hydrodynamics, meteorology, geology, certain branches of chemical technology (explosives, petroleum, and fuels, for example) and other disciplines adjacent to chemistry and physics, a thorough expert of chemical industry and industry in general, and an original thinker in the field of economy.”
Mendeleev was a co-founder of the Russian Chemical Society in 1869. He is given credit for introducing the metric system to the Russian Empire.
He studied the origin of petroleum and concluded that hydrocarbons are abiogenic (not produced by the action of living organisms) and form deep down below the Earth’s surface.
Regarding petroleum, Mendeleev wrote:
“The capital fact to note is that petroleum was born in the depths of the earth, and it is only there that we must seek its origin.”
Video – Dmitri Mendeleev
This video introduces us to the man behind the Periodic Table – the brilliant Russian chemist Dmitri Mendeleev.

