The ozone hole refers to the excessive thinning of the ozone layer in a region. This happens mainly in the winter to ozone layers in high altitudes. The ozone hole is due to the chemical action of CFCs and other pollutants in the atmosphere. CFCs stands for chlorofluorocarbons.
Chlorofluorocarbon molecules contain chlorine, carbon, and fluorine. Fluorocarbon molecules contain fluorine and carbon. Fluorocarbons also deplete ozone levels.
Ozone at high altitudes protects life on the Earth’s surface from the effects of harmful Sun rays. Specifically, ultraviolet rays. When there is an ozone hole, more UV rays get through, which for humans increases the risk of developing skin cancer. UV stands for ultraviolet.
What is ozone?
Ozone or O3 is a gas which consists of three oxygen atoms. The gas exists in trace amounts in the stratosphere. The stratosphere is the second atmospheric layer of our atmosphere, just above the troposphere. The stratosphere extends from 8 miles to 30 miles (12.8 to 48.8 km) above ground level.
Ozone protects humans from the Sun’s UV radiation. In fact, it protects all forms of life on Earth.
Chemical reactions from pollutants in the troposphere lead to the creation of O3. Vehicle exhaust and gasoline vapors are examples of such pollutants.
Ozone, however, is not always beneficial to humans. At ground level, high ozone levels are bad for the health of humans, other animals, and plants.
High O3 levels at ground level can exacerbate asthma and emphysema symptoms. They can also cause lung and throat irritation.
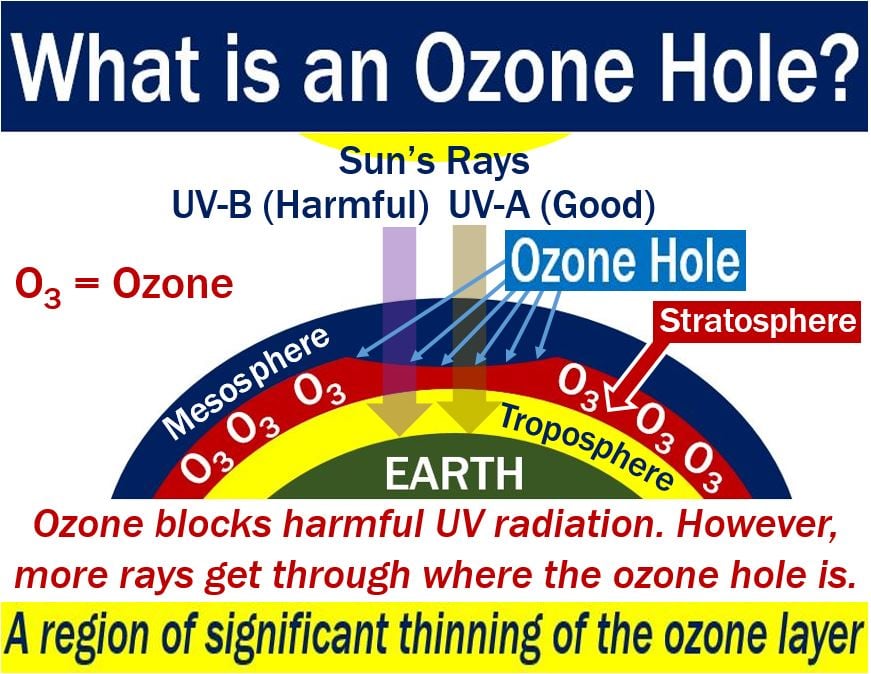
What causes an ozone hole?
In 1985, researchers discovered that over Antarctica, ozone levels were lower. They said that certain substances had destroyed ozone when they reacted with it.
We know that, for example, when nitrogen, chlorine, bromine, and hydrogen come into contact with O3, they destroy it.
However, there are some other substances that human activity has created which also destroy ozone. For example, we are responsible for the emission of CFCs into the atmosphere. CFCs destroy ozone, i.e., they are ozone hole creators.
CFCs break down in UV light to release reactive chlorine atoms. The chlorine subsequently reacts with ozone and destroys it.
We once used CFCs in aerosol spray-cans and for making insulating foam. CFCs made their way into the atmosphere, eventually reaching the ozone layer. Once there, the chances of creating an ozone hole are much greater.
We do not use CFCs any longer. We now use chemicals that are not associated with ozone hole risk.
Ozone hole is shrinking
In an article in LiveScience, Stephanie Pappas writes that the ozone hole over Antarctica is smaller. In fact, it has shrunk to the smallest it has been since 1988.
However, scientists say that the smaller ozone hole is not a sign that it is healing.
According to Ms. Pappas:
“It [ozone hole shrinking] is a side effect of the Antarctic vortex – a low-pressure system that rotates clockwise above the southernmost continent – undergoing a few years of instability and warmth, which prevented the proliferation of polar stratospheric clouds.”
